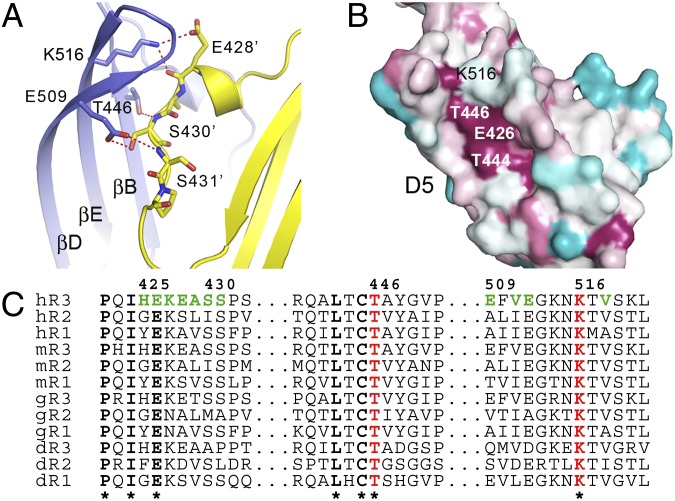
Fig. 2.
VEGFR-3 D5 homotypic interactions are centered to the conserved residues Thr446 and Lys516. (A) Homotypic interactions in D5. The two chains are shown in cartoon form, color-coded as in Fig. 1. Thr446, Glu509, Lys516, and their counterparts in strands A and A’ are shown as sticks. Hydrogen bonds are shown as red dashed lines. (B) D5, with conservation pattern in cyan through dark red for variable to conserved amino acids. Evolutionary rates of human, mouse, chicken, and zebrafish VEGFR sequences were were plotted using the ConSurf Web server. Thr444, Thr446, Glu426, and Lys516 compose a highly conserved patch on the D5 surface. (C) Representative amino acid sequence alignment from B. Residues involved in homotypic interactions are colored.