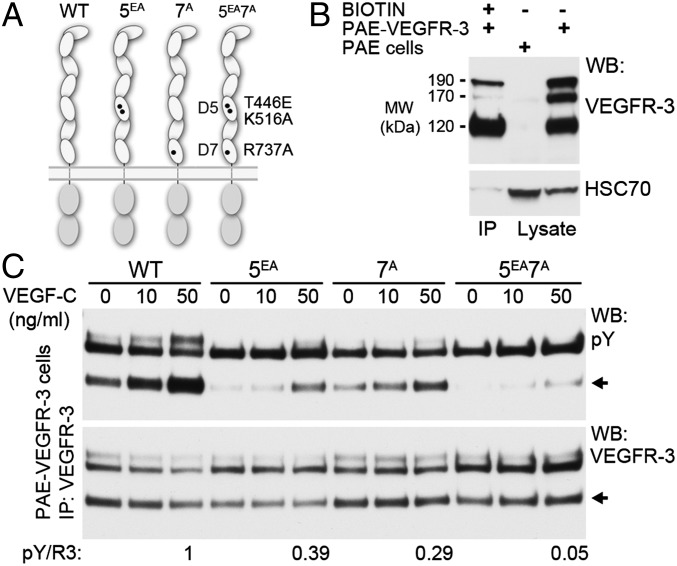
Fig. 3.
Homotypic interactions in D5 and D7 are mutually important for ligand-induced VEGFR-3 activation. (A) Schematic presentation of the WT VEGFR-3, 5EA, 7A, and 5EA7A mutants of VEGFR-3. (B) Biotinylation of cell surface-expressed VEGFR-3 isoforms. Biotinylated PAE-VEGFR-3 cell lysates were immunoprecipitated with streptavidin beads and blotted for VEGFR-3 and HSC70. Total lysates of PAE and PAE-VEGFR-3 cells were used as controls. (C) PAE cells stably expressing the VEGFR-3 constructs were stimulated with VEGF-C, and the cell lysates were immunoprecipitated with anti–VEGFR-3 and analyzed for VEGFR-3 autophosphorylation (pY) and expression (R3) by Western blot analysis. The pY/R3 ratio was quantified based on the ∼120-kDa band representing the fully processed VEGFR-3 (6, 7).