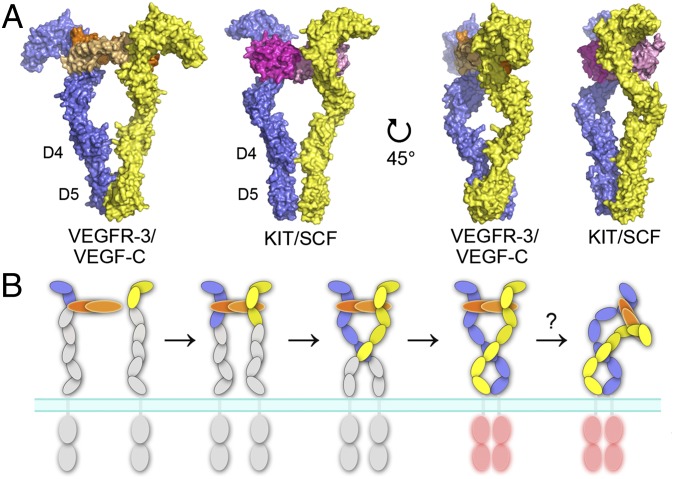
Fig. 6.
The mechanism of ligand-induced VEGFR dimerization and activation. (A) Comparison of the ligand-induced dimerization of D1-5 of type III/V RTKs. The KIT/SCF complex (22) and the model of the VEGFR-3/VEGF-C complex are shown as surface representations in two orientations. SCF dimer is colored in magenta and in light magenta, and the two chains of KIT, VEGF-C, and VEGFR-3 are color-coded as in Fig. 1. (B) A proposed model of the ligand-induced dimerization and activation of VEGFRs. D1-2 represents the major ligand-binding unit. Ligand-induced D2-3 reconfiguration facilitates homotypic interactions in D5 and D7 that together are important for VEGFR activation. SAXS data indicates bending of the VEGF-C/VEGFR-3 complex around D3-5.