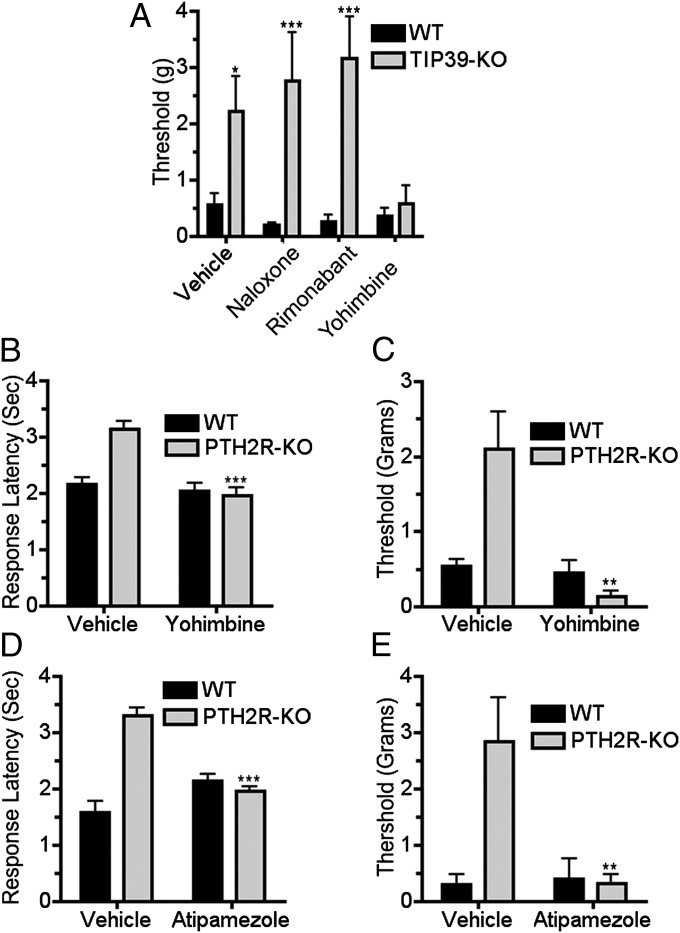
Fig. 4.
α-2 adrenergic block reverses the apparent recovery of nerve-injured TIP39-KO and PTH2R-KO mice. The opioid antagonist naloxone (5 mg/kg i.p.) and the cannabinoid antagonist rimonabant (5 mg/kg i.p.) did not change the mechanical sensitivity of TIP39-KO mice 60 d after nerve injury, but the adrenergic blocker yohimbine (2.5 mg/kg i.p.) decreased the mechanical threshold to the WT level (A; F3, 49 = 15.4, P < 0.05 for interaction, F1, 49 = 47.2, P < 0.001 for genotype and F3, 49 = 12.9, P < 0.05 for treatment as measured by two-way ANOVA. Bonferroni post hoc test showed significant differences between “no treatment” groups, P < 0.05, “naloxone” P < 0.001, and “rimonabant” groups P < 0.001 but not between “yohimbine” treated groups, P > 0.05). Thermal and mechanical withdrawal thresholds of WT and PTH2R-KO mice were also evaluated 40 d following PNL with, or without, α-2 adrenergic antagonist administration. Yohimbine (2.5 mg/kg i.p.) increased the thermal (B; F1, 44 = 14.5, P < 0.001) and mechanical (C; F1, 44 = 10.4, P < 0.01) sensitivity of injured PTH2R-KO mice, bringing it to the level of neuropathic WT. Intrathecal α-2 adrenergic receptor blocker (atipamezole, 0.3 mg/kg) produced a similar reversal of the apparent recovery of PTH2R-KO mice from thermal (D; F1, 18 = 43.7, P < 0.001) and mechanical hypersensitivity (E; F1, 18 = 9.4, P < 0.01).