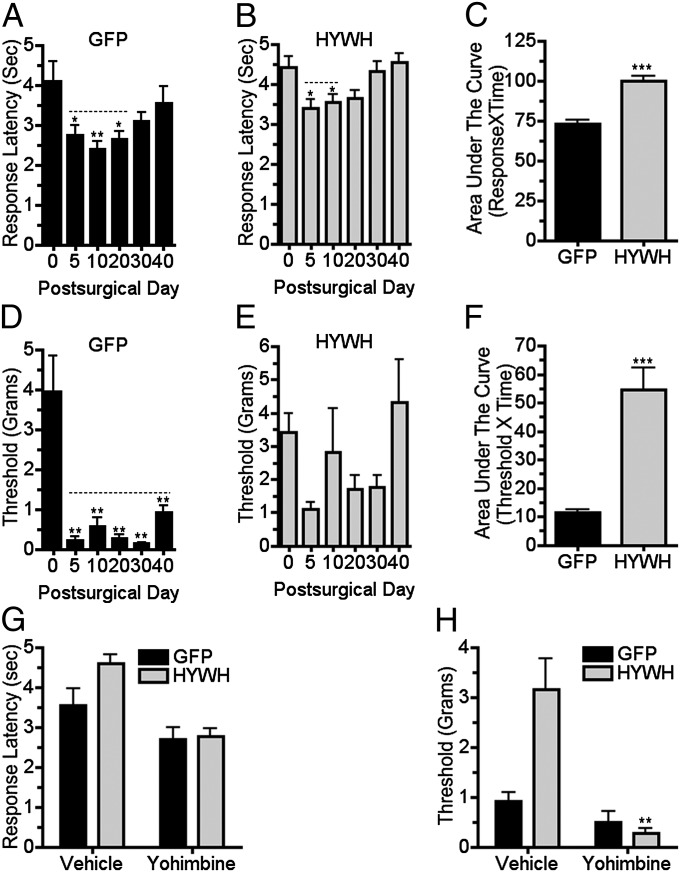
Fig. 5.
Brainstem delivery of a PTH2R antagonist reduces development of mechanical hypersensitivity following nerve injury. Thermal and mechanical withdrawal thresholds were measured following PNL in mice with LC-targeted injection of a PTH2R antagonist-coding virus. Control virus (GFP) injected mice had increased thermal sensitivity (A; F5, 41 = 3.9; P < 0.01) that was significantly different from their presurgical sensitivity for 30 d following surgery. Mice injected with antagonist encoding virus (HYWH) had increased thermal sensitivity (B; F5, 83 = 4.9; P < 0.001) for only 20 d. Increased mechanical sensitivity after PNL in control virus-injected mice (D; F5, 41 = 14.4; P < 0.001) lasted for 40 d but was not significant in HYWH-injected mice (E; F5, 83 = 2.2; P > 0.05). Area under the curve measurements over the 40-d experimental period indicated significant differences between thermal (C; P < 0.001, n = 7–14 per group) and mechanical (F; P < 0.001, n = 7–14 per group) sensitivities of mice injected with the control and antagonist viruses. Yohimbine administration (2.5 mg/kg i.p.), 40 d following PNL, significantly increased the thermal (G; F1, 38 = 21.7, P < 0.001) and mechanical (H; F1, 38 = 14.1, P < 0.001) sensitivity of mice injected with antagonist virus before surgery, bringing their sensitivity to the level of control virus-injected neuropathic mice. Statistical comparison showed significant interaction between virus and drug administration only for the mechanical thresholds (H; F1, 38 = 7.8, P < 0.01). P values in legend refer to effect of time and symbols above the columns refer to post hoc tests. For drug treatments, P values in the legend refer to virus × drug interaction. *P < 0.05, **P < 0.01, ***P < 0.001.