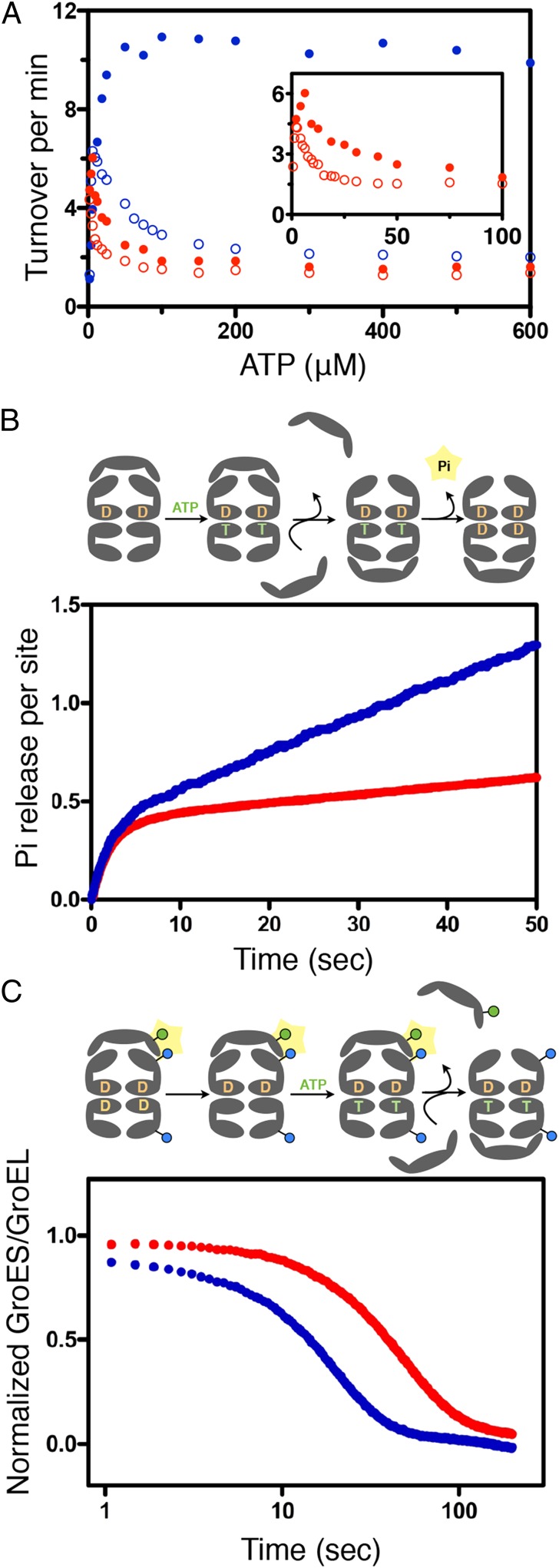
Fig. 2.
Functional properties of GroELWT and the GroELD83A/R197A. (A) Steady state hydrolysis of ATP as a function of [ATP] by GroELWT (blue) or GroELD83A/R197A (red) measured as previously described in the absence (open dots) or presence (filled dots) of denatured α-lactalbumin (3.6 μM). These measurements were conducted at 37 °C with 100 mM K+ and 10 mM Mg2+. (B) Presteady state hydrolysis of ATP by GroELWT (blue) or GroELD83A/R197A (red) monitored by fluorescence of phosphate binding protein labeled with N-[2-(1-maleimidyl)ethyl]-7-(diethylamino)coumarin-3-carboxamide. The accepting state complex [cisGroEL-ADP-GroES][transGroEL] was mixed with ATP and labeled PBP to initiate the reaction. The rate constant for the burst phase was 0.49 s−1 (GroELWT) and 0.45 s−1 (GroELD83A/R197A). These measurements were conducted at 37 °C with 200 mM K+ and 10 mM Mg2+. (C) Dissociation of GroES from the cis ring of GroELE315C-IAEDANS (blue) or GroELD83A/R197A/E315C-IAEDANS (red) monitored by decrease of FRET signal. The resting state complex [cisGroELIAEDANS-ADP-GroESF5M][transGroELIAEDANS-ADP] was mixed with ATP and the excess amount of unlabeled GroES to initiate the reaction. The mean residence of GroESF5M dissociating from the wild type GroELIAEDANS resting state was ∼12 s and the D83A/R197A GroELIAEDANS resting state was ∼37 s. These measurements were conducted at 37 °C, 200 mM K+, and 10 mM Mg2+.