Table 1.
Kinetic parameters for hydrolysis of Group I paraoxon analogs by rePON1 G2E6
| Molecule ID |
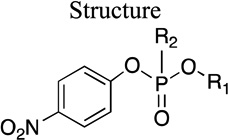 |
kcat s−1 |
KM mM |
kcat/KM M−1 s−1 |
IC50 mM |
|
|---|---|---|---|---|---|---|
| R1 | R2 | |||||
| 1 | Et | OEt | 2.1 ± 0.2 | 0.9 ± 0.2 | 2,300 ± 700 | |
| 6 | Me | OMe | > 0.2 | > 1.5 | 100 ± 10 a | |
| 7 | Et | OMe | 1.5 ± 0.1 | 0.6 ± 0.2 | 2,800 ± 800 | |
| 8 | Et | OPr | 0.63 ± 0.02 | 0.4 ± 0.1 | 1,700 ± 200 | |
| 9 | Et | Oi-Pr | 0.14 ± 0.01 | 0.6 ± 0.1 | 230 ± 50 | |
| 10 | Pr | OPr | 0.07 ± 0.01 | 0.3 ± 0.1 | 200 ± 100 | weak b |
| 11 | i-Pr | Oi-Pr | c | c | c | weak b |
| 12 | MeCyp | OMeCyp | weak | weak | weak | d |
| 13 | Bu | OBu | c | c | c | d |
| 14 | Et | OPentyl | 0.18 ± 0.04 | 1.3 ± 0.7 | 140 ± 80 | weak b |
| 15 | Et | OCyclohexyl | c | c | c | 0.5 b |
| 16 | Et | c | c | c | d | |
Saturation was not possible for this substrate under the experimental conditions; only kcat/KM could be determined via a linear fit.
Although the leaving group is the same as for paraoxon for all Group I compounds, we are able to observe inhibition of paraoxonase activity in substrates with turnover that is much slower than the turnover of paraoxon (1).
No observable activity.
No inhibition was observed.
