Abstract
Peripheral immune activation by bacterial mimics or live replicating pathogens is well known to induce central nervous system activation. Sickness behavior alterations are often associated with inflammation-induced increases in peripheral proinflammatory cytokines (eg, interleukin [IL]-1β and IL-6). However, most researchers have used acute high dose endotoxin/bacterial challenges to observe these outcomes. Using this methodology may pose inherent risks in the translational interpretation of the experimental data in these studies. Studies using Escherichia coli have yet to establish the full kinetics of repeated E. coli peripheral injections. Therefore, we sought to examine the effects of repeated low dose E. coli on sickness behavior and local peripheral inflammation in the open field test. Results from the current experiments showed a behavioral dose response, where increased amounts of E. coli resulted in correspondingly increased sickness behavior. Furthermore, animals that received a subthreshold dose (ie, one that did not cause sickness behavior) of E. coli 24 hours prior were able to withstand a larger dose of E. coli on the second day (a dose that would normally cause sickness behavior in mice without prior exposure) without inducing sickness behavior. In addition, animals that received escalating subthreshold doses of E. coli on days 1 and 2 behaviorally tolerated a dose of E. coli 25 times higher than what would normally cause sickness behavior if given acutely. Lastly, increased levels of E. coli caused increased IL-6 and IL-1β protein expression in the peritoneal cavity, and this increase was blocked by administering a subthreshold dose of E. coli 24 hours prior. These data show that progressive challenges with subthreshold levels of E. coli may obviate the induction of sickness behavior and proinflammatory cytokine expression.
Keywords: open field, E. coli, sickness behavior, repeated administration
Introduction
It is well known that activation of the peripheral immune system by bacterial mimics (eg, lipopolysaccharide [LPS]) or bacteria itself (eg, live Escherichia coli) induces robust behavioral alterations.1,2 These changes have been collectively termed “sickness behavior”, which include, but are not limited to, anhedonia, fever, increased slow wave sleep, decreased food and water intake, hyperalgesia, and lethargy. Along with these changes, decreased locomotion is one of the most widely measured sickness behaviors associated with immune activation/inflammation.3
To date, most researchers have used relatively high acute doses of endotoxin or bacteria to examine peripheral inflammation-induced behavioral consequences (ie, sickness behavior). However, the design of these experiments may have inherent conceptual limitations. For example, exposure to high levels of bacteria with a sudden onset may occur under limited circumstances such as sepsis, whereas exposure to low or asymptomatic levels of bacteria could be the more common events underlying the pathogenesis of many chronic diseases. In addition, repeated injection of certain bacteria has been used to effectively treat cancers and tumors in human patients.4–6 Such treatments are often designed to inject doses of anticancer bacteria at low levels to avoid severe symptoms of sickness. However, occasional outbreaks of sickness symptoms (eg, fever, hyperalgesia) during these treatments may threaten the completion of the treatment.7 Thus, understanding how repeated exposure to low levels of bacteria influence sickness symptoms has important clinical implications. Additionally, a recent report from our laboratory has shown consecutive low dose LPS injections caused behavioral sensitization that was coupled with cFos activation in areas associated with sickness symptoms,8 as opposed to high doses of LPS that often cause endotoxin tolerance. Therefore, it is important to ascertain whether repeated injections of bacteria containing LPS would also cause exacerbated sickness behavior.
In the current report, we investigated: (1) dose-dependent behavioral responses to increasing numbers of bacteria following a single injection of E. coli in both male and female mice; (2) the effect of a subsequent injection of the same and/or increasing dose(s) of E. coli on sickness behavior following a subthreshold bacterial challenge on the previous day in male mice; and (3) the impact that these subthreshold doses of E. coli, that did not cause sickness behavior, had on the local inflammatory cytokine production again in male mice. Results indicated that male animals were able to behaviorally tolerate (ie, not show signs of sickness behavior) progressively increased doses of E. coli if the prior day(s) injection failed to induce a sickness response. Furthermore, an otherwise robust local proinflammatory cytokine expression was diminished if male animals received a subthreshold dose of E. coli injection on the previous day. Opposed to the behavioral and immunological kinetics following LPS administration, which indicate a sensitization following low dose LPS administration, the current data indicated male animals showed behavioral and immunological desensitization if they were given a subthreshold administration of E. coli on the previous day(s).
Methods
Subjects
Subjects were 6 to 8 week-old male and female inbred FVB mice purchased from Charles River Laboratories International Inc (Wilmington, MA, USA). Animals were allowed to acclimate in the animal facility for ~1 week prior to experimental procedures. Mice were group housed 5/cage in standard polycarbonate mouse cages in an American Association of Accreditation of Laboratory Animal Care (AAALAC) facility, and maintained on a 12 hour light/dark cycle with lights being turned on at 6 am. Animals had free access to food and water except for at brief times in which they were removed from their home cages for intraperitoneal (ip) injections and behavioral tests. All the behavioral tests were conducted between 2 pm and 6 pm. All experiments were performed in conformity to a protocol approved by the Institutional Laboratory Animal Care and Use Committee (ILACUC) at The Ohio State University.
E. coli culture
The bacteria strain LT004 (kindly provided by Dr Monica Rydén Aulin of the Karolinska Institute, Solna, Sweden) was a genetically modified clinical uropathogenic E. coli strain (UPEC CFT073), containing a gfp+ DNA fragment insertion in the chromosomal cobS gene. The GFP was constitutively expressed under the tetracycline promoter PLtetO–1 Bacteria were cultivated in Luria-Bertani medium (USB Corporation, Cleveland OH, USA) at 37°C with chloramphenicol (Cm, 20 μg/mL) and diluted to OD600 reading of 0.1 in phosphate buffered saline (PBS), which corresponds to 2.21 × 108 cfu/mL, based on previously determined standard curve for E. coli. Higher or lower cfu of bacteria was then concentrated or diluted from the OD600 0.1 stock.
Open field
The open field test was conducted as previously described by Walsh and Cummins9 to evaluate the locomotor activity 3 hours after ip administration of E. coli LT004. The open field is a widely accepted behavioral test that requires no “learning” of novel tasks and has been shown to be extremely sensitive to immunological/inflammatory manipulations.8 Animals with obvious abnormal behavior (ie, 2 times the standard deviation) were excluded. Mice were not habituated to the open field apparatus or the test room before the initial exposure on day 1. Animals received single or repeated ip injections of E. coli or sterile PBS. Testing was conducted 3 hours after every injection. Animals were gently placed in our open field apparatus (40 × 40 × 25 cm Plexiglas® [Evonik Industries AG, Essen, Germany] box) and left to explore the arena for 5 minutes. The total distance travelled was automatically captured and analyzed using a digital video recording camera attached to a computer containing a video tracking software template (Ethovision 8.0; Noldus, Leesburg, VA, USA). For all animals exposed to E. coli more than once, total distance travelled was monitored 3 hours after every injection on all days.
Peritoneal cavity lavage and cytokine measurements
Immediately following behavioral assessment in open field, mice were euthanized by CO2 asphyxiation and peritoneal exudates were collected by lavage with Hanks’ Balanced Salt Solution (HBSS) (Invitrogen, Grand Island, NY, USA). Specifically, a small 0.5 cm incision was made in the peritoneal wall and the cavity was flushed with 1 mL of sterile HBSS following gentle massage. Peritoneal lavage fluid was centrifuged at 2000 rpm and supernatants were removed for further analyses of interleukin (IL)-1β and IL-6 protein levels by enzyme-linked immunosorbent assay (ELISA; BD Biosciences, San Jose, CA, USA) per manufacturer’s protocol.
Statistical procedures
Standard one-way ANOVAs were used to analyze all open field behavioral data and ELISA data. E. coli treatment and sex (on initial experiments; see Results section.) were used as the between-subjects variables. Significant main effects were subjected to Fisher’s PLSD post hoc analyses for further comparison. An alpha level of P < 0.05 was the criterion for rejection of the null hypothesis. Analyses were conducted using GraphPad Prism version 5.0 for Windows (GraphPad Software, Inc, La Jolla, CA, USA). Data are presented as treatment means ± standard error of the mean (SEM).
Results
Effects of varying acute doses of peripherial E. coli administration on open field activity in both male and female mice
Open field behavioral tests were performed 3 hours after male FVB mice received a single intrapeitoneal injection of varying concentrations of E. coli LT004. Results showed a main effect of E. coli treatment for total distance moved in the open field box (F(4,70) = 8.40; P < 0.05; see Figure 1A), where E. coli treated animals had a reduction in the total distance traveled when compared to PBS treated animals. Fisher’s post hoc analyses revealed that a significant decrease in the total distance traveled was found after the E. coli dose reached 4.0 × 107 cfu (considered the threshold to initiate sickness behavior in the present study) or greater compared to PBS treated controls (P < 0.05).
Figure 1.
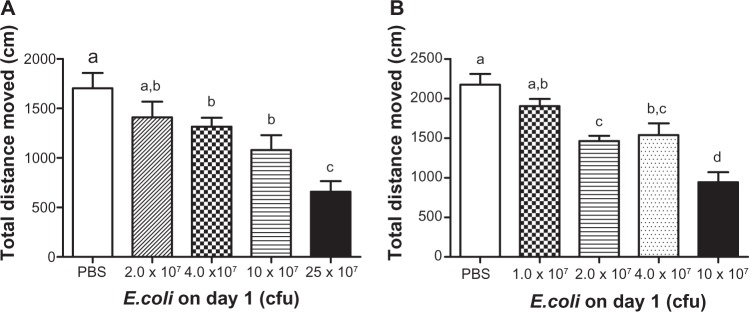
Open field activity following increasing doses of E. coli in male and female mice.
Notes: Mean total distance moved of male (A) and female (B) FVB mice in the open field 3 hours after a single ip injection of different concentrations of E. coli. Bars represent group means ± SEM. Different letters above each bar indicate significant differences among groups (P < 0.05), otherwise group means are not significantly different from each other.
Abbreviations: cfu, colony forming unit; E. coli, Escherichia coli; ip, intraperitoneal; PBS, phosphate buffered saline; SEM, standard error of the mean.
Similar experiments were also conducted in female mice. Open field behavioral results in female animals showed a significant main effect of E. coli treatment (F(4,60) = 15.30; P < 0.05; see Figure 1B), where E. coli treated animals had a reduction in the total distance traveled when compared to controls. Post hoc analyses indicated that a E. coli dose of 2.0 × 107 cfu or greater induced sickness behavior compared to PBS treated animals by reducing activity in the open field (P < 0.05). Compared to male mice, female animals had higher baseline locomotor activity (2174 ± 134.2 cm versus 1702 ± 154.7 cm, P < 0.05), and were more sensitive to E. coli-induced behavioral alterations, as they showed sickness behavior when injected with a lower dose of E. coli (sickness behavior was obverved after 2 × 107 cfu E.coli was injected in female, but not male animals).
Open field behavioral effects following two consecutive days of peripheral E. coli administration in male mice
Previously our laboratory has shown 2 days of 1 μg/kg LPS injections had a sensitization effect on open field activity, where repeated administration of LPS induced a greater sickness behavior response in male mice.8 Therefore, experiments were conducted to determine the effects of repeated E. coli injections over a 2 day peroid in male mice. After receiving 2.0 × 107 cfu E. coli injection on day 1, which was considered a subthreshold dose that did not induce significant sickness behavior on the first day, animals were given varying amounts of E. coli on day 2. Current data indicate that animals pretreated with 2.0 × 107 cfu E. coli on day 1 were refractory to the E. coli challenge on day 2. Results show there was a significant main effect of E. coli treatment (F(3,45) = 5.98; P < 0.05; see Figure 2), where animals treated with E. coli had a reduction in the total distance traveled compared to controls. Fisher’s post hoc analyses indicated the administration of 2.0 × 107 cfu E. coli to animals on day 1 and 25 × 107 cfu E. coli on day 2, which induced robust sickness behavior if given acutely (Figure 1A), or repeated administration of 2.0 × 107 cfu E. coli on day 1 and 2, failed to cause a significant reduction in the total distance traveled (P < 0.05; see Figure 2). Therefore, a subthreshold dose of E. coli offered a protective effect against a subsequent 2.0 × 107 cfu and 25 × 107 cfu E. coli challenge the following day. Nevertheless, if the dose was increased to 50 × 107 cfu, animals exhibited sickness behavior evidenced by a significant reduction in the total distance moved compared to the PBS, 2.0 × 107 cfu, and 25 × 107 cfu E. coli treated groups (P < 0.05).
Figure 2.
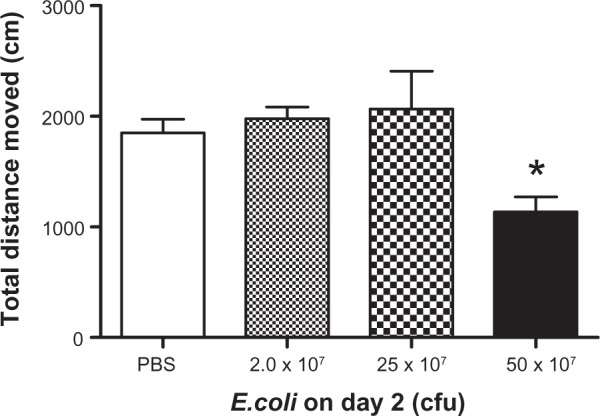
Open field activity following two days of repeated E. coli challenge.
Notes: Mean total distance moved in the open field 3 hours following a second ip injection of varying concentrations of E. coli 24 hours after receiving 2 × 107 E. coli injections in male mice. Bars represent group means ± SEM. *Indicates a significant difference compared with PBS control group (P < 0.05).
Abbreviations: cfu, colony forming unit; E. coli, Escherichia coli; ip, intraperitoneal; PBS, phosphate buffered saline.
Effects of consecutive E. coli injections on peritoneal lavage fluid Il-1β and Il-6 expression in male mice
Increased peripheral proinflammatory cytokines have been highly correlative to alterations in sickness behavior; therefore peritoneal lavage fluid levels of IL-1β and IL-6 were measured in mice subjected to a single or consecutive E. coli challenge(s). Consistent with the patttern of sickness behavior in the open field IL-1β and IL-6 protein levels exhibited a dose dependent increase resulting from acute E. coli administration in male mice. Data revealed significant main effects of E. coli treatment, where E. coli treated animals had increased IL-1β and IL-6 protein expression compared to controls (F(3,16) = 6.35, F(3,16) = 27.59, respectively; P < 0.05; see Figure 3A and B). Post hoc analyses indicated that increased protein expression was detected following a single injection of 10 × 107 cfu and 25 × 107 cfu E. coli for both IL-1β (P < 0.05; see Figure 3A) and IL-6 (P < 0.05; see Figure 3B) compared to PBS treated animals. Importantly, male animals that received a single 2.0 × 107 cfu E. coli injection on day 1, followed by a 25 × 107 cfu E. coli injection on day 2, had undetectable IL-1 β and very low IL-6 protein levels.
Figure 3.
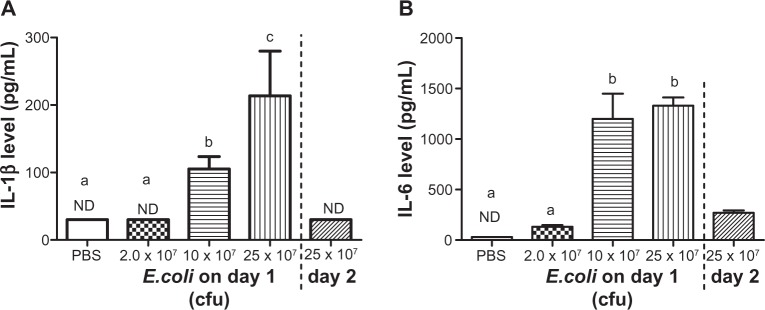
Peritoneal IL-1 β and Il-6 protein levels following a single or repeated E. coli challenge.
Notes: (Left of dashed line) Mean peritoneal fluid Il-1 β (A) and Il-6 levels (B) 3 hours after a single injection of varying concentrations of E. coli in male mice. (Right of dashed line) Mean peritoneal fluid Il-1 β (A) and Il-6 levels (B) 3 hours after receiving a 25 × 107 E. coli injection following 2.0 × 107 E. coli injection 24 hours before in male mice. Bars represent group means ± SEM. Different letters above each bar indicates significant differences among groups (P < 0.05), otherwise group means are not significantly different from each other.
Abbreviations: cfu, colony forming unit; E. coli, Escherichia coli ; IL, interleukin; ND, not detectible; PBS, phosphate buffered saline; SEM, standard error of the mean.
Open field behavior effects following three consecutive days of peripheral E. coli administration in male mice
To further explore the protective effects of sub-threshold E. coli injections on subsequent E. coli challenges, we designed a 3-day E. coli injection paradigm to see whether sub-threshold E. coli challenges on days 1 and 2 could also serve a protective effect to an even higher E. coli challenge on day 3. Male mice received 2.0 × 107 cfu E. coli on day 1 and 25 × 107 cfu E. coli on day 2; a regimen that was perviously shown not to induce sickness behavior (see Figure 2). On day 3, differing amounts of E. coli were administered and 3 hours later open field activity was monitored. Control mice received 3 days of PBS injections. Data revealed a significant main effect of E. coli treatment, where E. coli treated animals had a reduction in the total distance traveled compared to PBS treated controls (F(2,35) = 10.16; P < 0.05; see Figure 4). Post hoc analyses showed no significant difference in total distance moved between 100 × 107 cfu E. coli group and control group, indicating that the animals could behaviorally “tolerate” 25 times the number of E. coli that induced sickness behavior after a single injection on day 1 (P = 0.18; see Figure 4A). Furthermore, although 100 × 107 cfu E. coli did not induce sickness behavior, a dose of 200 × 107 cfu E. coli induced a slight reduction in the total distance traveled on day 3 when compared to PBS treated control animals (P < 0.05; see Figure 4). These data indicate that our 2-day behavioral tolerance effect to subthreshold E. coli challenges can have an even more robust effect if extended to a third day in male subjects. In addition, injection of 100 × 107 cfu E.coli on the first day caused piloerection in all the tested animals, whereas this dose of E.coli did not induce any piloerection on the third day of this treatment regiment.
Figure 4.
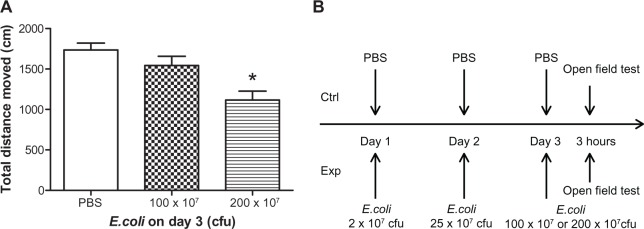
Open field activity following three days of repeated E. coli challenge.
Notes: (A) Mean total distance moved in the open field 3 hours after different concentrations of E. coli injections on day 3 in male mice. All E. coli treated mice received 2 × 107 E. coli on day 1 and 25 × 107 E. coli on day 2. Control mice received PBS for 3 days. Bars represent group means ± SEM. *Indicates a significant difference compared with PBS control group (P < 0.05). (B) Schematic of the 3 day experimental design.
Abbreviations: cfu, colony forming unit; Ctrl, control; Exp, experimental animals; E. coli, Escherichia coli; PBS, phosphate buffered saline; SEM, standard error of the mean.
Discussion
Behavioral alterations following peripheral immune stimulation with either live replicating bacteria or bacterial mimics (eg, LPS) have been well established. The subsequent release of proinflammatory cytokines such as IL-1 and IL-6 by immune cells in the periphery have been discovered to be major responses behind immune-to-brain signaling.10 The primary behavioral alterations found following peripheral immune stimulation are collectively termed “sickness behavior”. Until recently, most researchers have relied on high acute doses of LPS and E. coli as inflammatory stimulants to study neuro-immune crosstalk. These methodologies are appropriate for studying severe acute inflammatory reactions, as in the case of septic shock, but have the potential to misrepresent the etiology of conditions such as affective and chronic inflammatory disorders. For example, these conditions do not have an acquired pathology similar to those found in high dose LPS or bacterial load studies; thus, a more comprehensive model/understanding is needed to fully understand the kinetics in these disorders.
As mentioned, sickness behavior is often induced by peripheral immune stimulation with E. coli. However, the kinetics and dosages are often overlooked and could have profound implications in the translational interpretation of these results. Our initial experiments were designed to determine the cfu/dose of E. coli that was able to induce sickness behavior in the open field test in both male and female animals. The open field is the most commonly used behavioral test to determine if immunological manipulations have affected mobility (ie, induced sickness behavior). Animals that have been given a peripheral immune challenge show a robust decrease in mobility which has been correlated to heightened activation of areas in the CNS related to sickness behavior such as the paraventricular nucleus, nucleus of the solitary tract, and ventral lateral medulla.11 Current data show that a single dose bacterial load of 4.0 × 107 cfu E. coli was sufficient to induce a decrease in open field activity in male FVB mice, and there was a further reduction following even higher doses. A single dose of 2.0 × 107 cfu E. coli in male FVB mice was insufficient to alter open field activity 3 hours after injection (see Figure 1A). A similar dose response pattern was found in female mice; however, the same single dose of 2.0 × 107 cfu E. coli in female mice induced a decrease in mobility in the open field (see Figure 1B). These data suggest that female mice were more sensitive to peripheral immune-induced stimulation of sickness behavior than their male counterparts. These data also align with previous studies from our laboratory and others that show female mice are behaviorally and biologically more sensitive than male mice following low dose peripheral LPS administration.8,12
Knowing a single dose of 2.0 × 107 cfu E. coli was insufficient to cause a decrease in open field activity in male mice, and based on previous studies conducted in our laboratory showing subthreshold doses of LPS given repeatedly perpetuate sickness behavior,8 we examined the effects of repeated subthreshold E. coli on sickness behavior in male mice. Results from current studies showed male animals that received 2.0 × 107 E. coli 24 hours before were able to withstand 25 × 107 E. coli on the second day without causing sickness behavior (see Figure 2). Comparing these data with prior reports, the sickness behavior response patterns differ between repeated subthreshold doses of LPS and E. coli.8 Additionally, data show that, although animals were able to withstand 25 × 107 cfu E. coli without inducing a sickness behavior response in the open field test 3 hours after the injection, they succumbed to sickness behavior after 50 × 107 cfu E. coli injection on the second day (see Figure 2). These data show that male animals are able to tolerate an E. coli dose 12.5 times higher than the previous dose without inducing sickness behavior if preceded by a behaviorally subthreshold dose of E. coli 24 hours prior.
Proinflammatory cytokines, namely IL-1β and IL-6, have long been implicated as the major mediators that the immune system uses to signal the CNS of peripheral inflammatory statuses. Therefore, we examined local IL-1β and IL-6 production in the peritoneal cavity as a possible mechanism behind the reduction in sickness behavior seen following repeated E. coli administration in male mice. Results show a similar dose response pattern as open field activity following a single dose of E. coli. Specifically, a single dose of 10 × 107 cfu or 25 × 107 cfu E. coli induced a robust increase in both IL-1β and IL-6 protein production in peritoneal lavage fluid 3 hours following injection, whereas 2.0 × 107 cfu E. coli did not (see Figure 3A and B). Furthermore, exposure to 25 × 107 cfu E. coli following a 2.0 × 107 cfu dose 24 hours prior failed to induce IL-1β and IL-6 production, as it did for sickness behavior in open field in male mice. These results indicate that the reduction in local proinflammatory cytokine production following repeated exposure to E. coli is a possible mechanism behind the lack of sickness behavior seen under the same conditions.
Lastly, to examine the robustness of perpetual increased/repeated E. coli injections on preventing sickness behavior, we extended this model to a third day of injections in male mice. Data from these sets of experiments indicated male mice that received 2.0 × 107 cfu E. coli on day 1 and 25 × 107 cfu E. coli on day 2 were able to behaviorally “tolerate” 100 × 107 cfu E. coli given on day 3 (ie, did not show a reduction in activity). This dose was 50 times greater than the dose given on day 1, and 4 times greater than the dose for day 2. If given as a single bolus injection, 100 × 107 E. coli would mimic septic conditions and cause a strong sickness behavior response (data not shown). However, behavior was not altered if exposed to subthreshold doses of E. coli that did not induce sickness behavior on previous days. These data show the robustness of this kinetic response and demonstrate unexpected dynamics compared to the responses induced by repeated injection of LPS.8
It should be noted that the present study only investigated one bacterial strain, in one strain of mice, using one behavioral test. In the literature, it is known that different strains of bacteria can cause different types of sickness symptoms. For example, staphylococcal enterotoxin A does not cause decreased activity in the open field, but induces neophobia.13 Different strains of mice are also known to produce different cytokine responses and behavioral changes after a given immune challenge.14,15 In addition, different aspects of the sickness behavior may be decoupled (eg, fever and sleep changes can be separated).16 Thus, the results of present study should not be overgeneralized. In addition, the 3 hour time point after E. coli injection was chosen to study the open field behavior because previous studies have found acute cytokine response and behavioral changes to E. coli at this time point.17 Whether the conclusion reached in the present study is valid for other time points remains to be confirmed. On the other hand, a previous study showed that reduced movement in the open field test can be observed in mice that received sub-pyrogenic levels of LPS,12 suggesting the specific test used in the current study may have predictive value for other less sensitive sickness symptoms. In addition, most sickness symptoms can be mediated by the inflammatory cytokines IL-1 and IL-6.10 Because we found that progressive administrations of subthreshold doses of E. coli reduced the induction of IL-1 and IL-6, it is possible that other IL-1- and IL-6-dependent sickness symptoms might follow similar kinetic patterns as the behavior and proinflammatory production observed in this study did. Indeed, previous studies have observed that repeated injection of vaccines progressively reduced febrile responses,18 similar to the current finding. Further, we noted that 100 × 107 cfu E. coli injected on day 1 produced piloerection in all the injected male mice, but failed to do so in any male mouse receiving 100 × 107 cfu E. coli on day 3 after prior subthreshold doses of E. coli. Thus, the pattern of changing sickness behavior we observed in the open field is not limited to the reduced activity alone.
As for the cellular and molecular mechanisms underlying the observed phenomenon, it is a matter of speculation. There are two relevant theories. The first is endotoxin tolerance. Numerous in vitro studies found monocytes exposed to LPS for the second time after a prior LPS stimulation became tolerant.18 The tolerant monocytes are not necessarily less responsive to LPS, but rather shift their production of proinflammatory cytokines (IL-1, IL-6, and TNFα) to anti-inflammatory cytokines (IL-10).18 In vivo studies of endotoxin tolerance typically involve a first exposure of animals to very high levels of endotoxin (eg, 5 mg/kg of LPS) followed by a second LPS or bacteria stimulation.19 The reason for the high levels LPS used in the first LPS stimulation is probably because only high levels of LPS stimulation can precondition most monocytes throughout the system for the manifestation of the subsequent general tolerance. This is not likely to have happened in the current study in which animals were injected with subthreshold levels of E. coli in the peritoneal cavity in their first exposure. While resident monocytes in the peritoneal cavity may become tolerant after the first E. coli injection, cells arriving after the second E. coli injection may not have been exposed to the E. coli on the first day and are therefore less likely to become tolerant. This theory also fails to explain our previous observation that ip injection of subthreshold doses of LPS sensitized, rather than desensitized, the animal’s behavioral response to low dose LPS administration.8 The second theory is a preferential activation theory. We speculate that subthreshold levels of E. coli injected on the first day might be preferentially taken up by innate immune cells, which causes the recruitment and subsequent activation of more innate immune cells to the peritoneal cavity, thus progressively amplifying the bactericidal and endotoxin-neutralizing innate immunity and obviates the need to produce sickness symptoms. This is different from injections of free LPS in our previous study in which the injected free LPS might indiscriminately stimulate both innate immune cells and many non-immune cells such as endothelial cells. These non-immune cells are unable to neutralize the endotoxin, but are capable of producing high levels of inflammatory cytokines to trigger sickness symptoms including HPA activation, which leads to suppression, rather than facilitation, of the function of innate immune cells. In support of this theory, it is known that injection of LPS encapsulated in liposomes, which are preferential taken up by macrophages, can reduce the potential for LPS to induce fever by 1000 fold, and sickness symptoms can be dramatically blocked after subsequent injections of LPS or bacteria by prior liposome-LPS treatment.20 Although this mechanism requires further confirmation and elucidation, the phenomenon uncovered in the present study nevertheless might help design bacterial treatments that could avoid the induction of sickness symptoms.
Overall, these data highlight for the first time important intricacies of behavioral responses induced in a model that mimics gradually increasing levels of bacterial infection. Furthermore, this report indicates the possibility that the innate immune system can be progressively “conditioned” for heightened immunological responses without inducing sickness behavior if care is taken not to over stimulate the system.
Acknowledgments
This work was supported in full by the NIH grants: RO1MH-093473-JFS, F32 DE-022230-AJT, and RO1AI-076926-NQ.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann N Y Acad Sci. 2001;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- 2.Quan N, Banks WA. Brain-immune communication pathways. Brain Behav Immun. 2007;21(6):727–735. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Abraham J, Johnson RW. Central inhibition of interleukin-1beta ameliorates sickness behavior in aged mice. Brain Behav Immun. 2009;23(3):396–401. doi: 10.1016/j.bbi.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poldervaart MT, Breugem CC, Speleman L, Pasmans S. Treatment of lymphatic malformations with OK-432 (Picibanil): review of the literature. J Craniofac Surg. 2009;20(4):1159–1162. doi: 10.1097/SCS.0b013e3181abb249. [DOI] [PubMed] [Google Scholar]
- 5.Kawamura M. Adjuvant immunotherapy for lung cancer. Nihon Rinsho. 2002;60(Suppl 5):459–462. [PubMed] [Google Scholar]
- 6.Ryan RM, Green J, Lewis CE. Use of bacteria in anti-cancer therapies. Bioessays. 2006;28(1):84–94. doi: 10.1002/bies.20336. [DOI] [PubMed] [Google Scholar]
- 7.Huang GT, Yang PM, Sheu JC, et al. Intratumor injection of OK-432 for the treatment of small hepatocellular carcinoma. Hepatogastroenterology. 1990;37(5):452–456. [PubMed] [Google Scholar]
- 8.Tarr AJ, Chen Q, Wang Y, Sheridan JF, Quan N. Neural and behavioral responses to low-grade inflammation. Behav Brain Res. 2012;235(2):334–341. doi: 10.1016/j.bbr.2012.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walsh RN, Cummins RA. The Open-Field Test: a critical review. Psychol Bull. 1976;83(3):482–504. [PubMed] [Google Scholar]
- 10.Kelley KW, Bluthé RM, Dantzer R, et al. Cytokine-induced sickness behavior. Brain Behav Immun. 2003;17(Suppl 1):S112–S118. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- 11.Lyte M, Li W, Opitz N, Gaykema RP, Goehler LE. Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiol Behav. 2006;89(3):350–357. doi: 10.1016/j.physbeh.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 12.Teeling JL, Felton LM, Deacon RM, Cunningham C, Rawlins JN, Perry VH. Sub-pyrogenic systemic inflammation impacts on brain and behavior, independent of cytokines. Brain Behav Immun. 2007;21(6):836–850. doi: 10.1016/j.bbi.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Kawashima N, Kusnecov AW. Effects of staphylococcal enterotoxin A on pituitary-adrenal activation and neophobic behavior in the C57BL/6 mouse. J Neuroimmunol. 2002;123(1–2):41–49. doi: 10.1016/s0165-5728(01)00486-6. [DOI] [PubMed] [Google Scholar]
- 14.Gibb J, Hayley S, Poulter MO, Anisman H. Effects of stressors and immune activating agents on peripheral and central cytokines in mouse strains that differ in stressor responsivity. Brain Behav Immun. 2011 Mar;25(3):468–482. doi: 10.1016/j.bbi.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Ferran C, Dy M, Sheehan K, et al. Inter-mouse strain differences in the in vivo anti-CD3 induced cytokine release. Clin Exp Immunol. 1991;86(3):537–543. doi: 10.1111/j.1365-2249.1991.tb02966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.García-García F, Yoshida H, Krueger JM. Interleukin-8 promotes non-rapid eye movement sleep in rabbits and rats. J Sleep Res. 2004;13(1):55–61. doi: 10.1111/j.1365-2869.2004.00386.x. [DOI] [PubMed] [Google Scholar]
- 17.Macarthur H, Couri DM, Wilken GH, et al. Modulation of serum cytokine levels by a novel superoxide dismutase mimetic, M40401, in an Escherichia coli model of septic shock: correlation with preserved circulating catecholamines. Crit Care Med. 2003;31(1):237–245. doi: 10.1097/00003246-200301000-00037. [DOI] [PubMed] [Google Scholar]
- 18.Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 2009;30(10):475–487. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Wheeler DS, Lahni PM, Denenberg AG, et al. Induction of endotoxin tolerance enhances bacterial clearance and survival in murine polymicrobial sepsis. Shock. 2008;30(3):267–273. doi: 10.1097/shk.0b013e318162c190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrov AB, Semenov BF, Vartanyan YP, et al. Toxicity and immunogenicity of Neisseria meningitidis lipopolysaccharide incorporated into liposomes. Infect Immun. 1992;60(9):3897–3903. doi: 10.1128/iai.60.9.3897-3903.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


