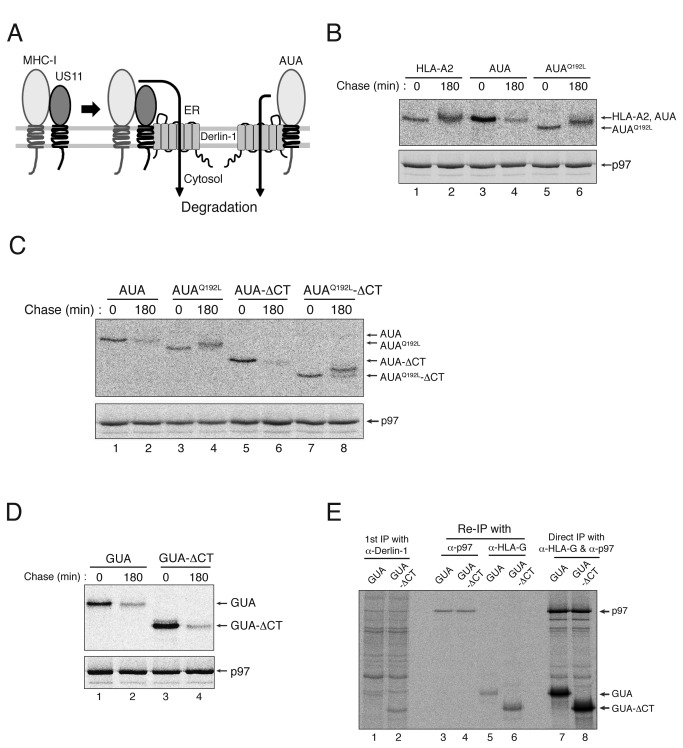
Figure 5. The MHC-I heavy chain cytosolic tail is not required for the degradation of MHC-I hybrids forced to interact with Derlin-1.
(A) A schematic representation of the degradation of an MHC-I/US11 hybrid forced to interact with Derlin-1. (B) AUA is degraded after binding to Derlin-1. HeLa cells were transfected with wild-type HLA-A2, AUA or AUAQ192L, metabolically labeled for 15 min, and chased for 0 or 180 min. HLA-A2, AUA or AUAQ192L was recovered by immunoprecipitation with mAb BB7.2, separated in SDS-PAGE gels, and analyzed by autoradiography. Immunoprecipitation of p97 (lower panel) indicates that the same amount of cell lysate was loaded into each lane of the gel. (C) The cytosolic tail of AUA is not required for its degradation. HeLa cells were transfected with AUA, AUAQ192L, AUA-ΔCT, or AUAQ192L-ΔCT, metabolically labeled for 15 min, and chased for 0 or 180 min. (D) Deletion of the MHC-I cytosolic tail does not block the degradation of GUA. HeLa cells were transfected with GUA or GUA-ΔCT, metabolically labeled for 15 min, and chased for 0 or 180 min. (E) Deleting the cytosolic tail from GUA does not abrogate its binding to Derlin-1. HeLa cells were transfected with GUA or GUA-ΔCT, metabolically labeled for 1 hr, lysed in 1% digitonin, and subjected to immunoprecipitation with an anti-Derlin-1 antibody. The precipitate was then boiled in SDS/DTT-containing buffer, diluted 10-fold in 1% NP-40, and then subjected to a second round of immunoprecipitation with the anti-p97 antibody or with mAb 4H84. All experiments were performed multiple times with similar results, and the data shown are representative of all results.