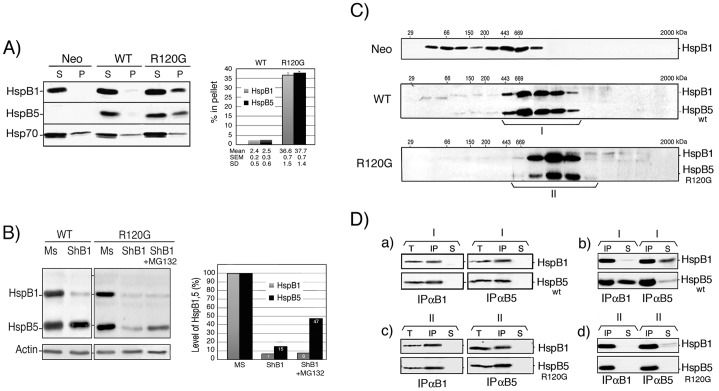
Figure 2. Characterization of HspB1, HspB5 and mutant HspB5 in HeLa cell clones.
A) Cellular distribution of HspB1, HspB5 (wild type and mutant) and Hsp70 upon cell lysis. Neo, WT and R120G cells were lysed in the presence of 0.1% Triton X-100 and spun at 10,000×g as described in Materials and Methods. The levels of HspB1, HspB5 and Hsp70 present in the supernatant and pellet fractions were detected in immunoblots probed with the corresponding antibodies (see Materials and Methods). Autoradiographs of ECL-revealed immunoblots are presented. Quantitative analysis of three independent experiments is presented in the adjacent figure. B) Effect of shRNA-mediated depletion of HspB1. WT and R120G cells were transiently transfected with control mismatch pSuperNeo-MsRNA27 (Mismatch: Ms) or pSuperNeo-ShRNA27 (ShB1) vector targeting HspB1 mRNA (see Materials and Methods). Two days after transfection, cells were analyzed in immunoblots probed with HspB1, HspB5 and actin antibodies. ShB1 transfected cells were also treated for the last 20 h before being analyzed with 0.5 µmol/l of MG132. Quantitative analysis of one particular experiment where the RNAi-mediated transient depletion of HspB1 was almost complete is presented in the adjacent figure. C) Analysis of HspB1 and HspB5 native sizes in Neo, WT and R120G cells. Cells were lysed as above and the 10,000×g cytosolic supernatant fractions were applied to Sepharose 6B gel filtration columns (see Materials and Methods). The presence of HspB1 and HspB5 in pooled fractions eluted from the columns was detected in immunoblots probed with the corresponding antibodies. Autoradiographs of ECL-revealed immunoblots are presented. 29, 66, 150, 200, 443, 669 kDa are gel filtration markers. Exclusion size of the column is 2000 kDa. Brackets indicate fractions that were pooled for further immunoprecipitation analysis. Size population I is from WT cells and size population II is from R120G cells. D) Co-immunoprecipitation studies. a) Size population I from WT cells was immunoprecipitated with either anti-HspB1 (IPαB1) or anti-HspB5 antibody (IPαB5). Immunoprecipitated proteins-bound to proteinG-sepharose were washed in IPP150 buffer containing 150 mM NaCl before being processed for gel electrophoresis. After migration in SDS-PAGE, proteins were revealed in immunoblots probed with either anti-HspB1 or anti-HspB5 antibody. T: aliquot of cytosolic supernatant fractions before immunoprecipitation, IP: immunoprecipitated proteins, S: aliquot from supernatant after immunoprecipitation. b) Same as a) except that washes of the immunoprecipitated proteins were performed in IPP300 buffer containing 300 mM NaCl. c–d) same as a–b) but in this case analysis was performed with size population II from R120G cells. Autoradiographs of ECL-revealed immunoblots are presented.