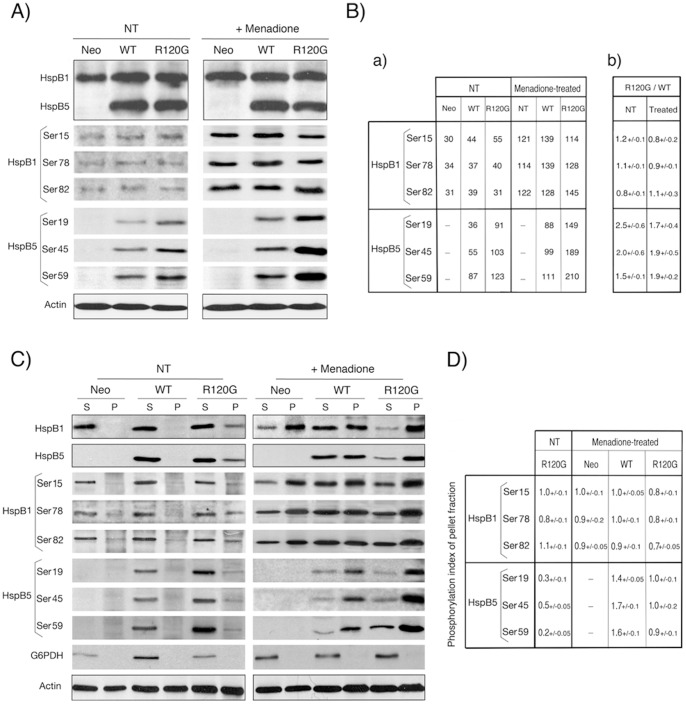
Figure 4. Analysis of HspB1 and HspB5 phosphorylation.
A) Immunoblot analysis of total cellular proteins. HeLa cells were either kept untreated (NT) or treated for 2 h with 60 µM menadione. Total cellular protein extracts were analyzed in immunoblots probed with antibodies that are specific for HspB1 or HspB5 and for HspB1 phosphorylation at either serine 15 (Ser15), 78 (Ser78) or 82 (Ser 82) or HspB5 phosphorylation at either serine 19 (Ser19), 45 (Ser45) or 59 (Ser59) (see Materials and Methods). The corresponding levels of total HspB1, HspB5 and actin are shown as controls (marked by a dark line surrounding immunoblots). Autoradiographs of ECL-revealed immunoblots are presented. B) Quantitative analysis of HspB1 and HspB5 phosphorylation in untreated cells and following exposure to menadione. Ba) Level of HspB1 and HspB5 phosphorylation in the immunoblot presented in A. The level of actin was used as standard of equivalent protein loading. Bb) Modulation of the phosphorylation of HspB1 and HspB5 by the R120G mutation. The R120G/WT ratio of the different HspB1 and HspB5 phosphoserine sites was defined as the ratio between the level of phosphorylation in R120G cells to that observed in WT cells. The R120G/WT ratios were calculated from three independent experiments. NT: non-treated. Treated: menadione-treated. Standard deviations are indicated (n = 3). Note the positive effect of the mutation towards HspB5 phosphorylation. C) Immunoblot analysis of fractionated cells. Same as A) but in this case 10,000×g supernatant (S) and pellet (P) fractions were analyzed from cells lysed in TEM buffer containing 0.1% Triton X-100 and spun at 10,000 g for 10 min. As in A, the corresponding levels of total HspB1, HspB5 and actin are shown as controls (marked by a dark line surrounding immunoblots). Autoradiographs of ECL-revealed immunoblots are presented. D) Quantitative analysis. The phosphorylation index of pellet fraction was defined as the ratio of the percentage of the phosphorylated protein in pellet to the percentage of the protein in that particular fraction. A value of 1.0 indicates that phosphorylation is proportional to the level of the protein in the pellet fraction. A value>1.0 is indicative of an enhanced phosphorylation of the protein in the insoluble fraction. A value<1.0 is indicative of a decreased phosphorylation of the protein in the insoluble fraction. Standard deviations are indicated (n = 3). Note the decreased phosphorylation of mutant HspB5 in the pellet of untreated R120G cells and the stimulated phosphorylation of pelleted wild type HspB5 in response to oxidative stress.