Abstract
When a person standing upright raises an arm on cue, muscles of the left and right sides of the body exhibit changes prior to and specific to the responding arm. We had standing participants perform a visual lexical decision task (“is this letter string a word?”), responding yes by raising one arm and no by raising the other arm. We recorded onset of the arm movement and onset of electromyographic activity in thigh, trunk, and shoulder muscles. We observed the expected responding arm specificity and found that the onset difference favoring word decisions was evident in similar magnitude at all measurement sites, with the difference at the levels of thigh, trunk and shoulder muscles available 225, 189, and 120 ms, respectively, prior to its manifestation at the level of arm movement. We discuss including (a) whole body reaction time along with event-related potentials in determining the decision-response, brain-body temporal relation, and (b) response execution along with response initiation in investigating mental chronometry.
A common experimental method for investigating visual word recognition is the lexical decision task. On presentation of a letter string the participant has to respond as quickly as possible whether the letter string is a word or a nonword, that is, to decide whether the letter string is an entry in the participant’s mental lexicon. Typically the participant is seated and the response is a key press, using one hand to press a yes key and one hand to press a no key. The well-known standard finding is that the reaction time (RT) to correctly decide yes is shorter than the RT to correctly decide no. Less well known is that factors presumed to influence the duration of mental events preceding the decision differentially influence the execution dynamics of the yes and no responses.
Patently, the latency between the onset of a letter string and a key press does not index the time taken to decide whether it is a word or not. That decision must occur some time prior to the key press. How much prior can, in theory, be determined by brain measures. Braun, Jacobs, Hahne et al. (2006) found that resolution of the yes decision in the measure of event-related potentials occurred at approximately 350 ms post-stimulus for an average yes key press latency of 659 ms.
The latency of a key press from a seated position may not, however, be a satisfactory index of when a lexical decision response becomes available. That is, it may give an inadequate appreciation of the brain-behavior temporal relation. Since Belenkii, Gurfinkel, and Paltsev (1967) and Paltsev and Elner (1967) it has been known that simple RT-like behavior when standing, such as raising an arm to an auditory signal, is preceded by subtle adjustments in the muscles of lower body segments that are specific to the arm movement and possibly related to the maintenance of posture. For example, if the right arm is raised rapidly in the forward direction, electromyographic (EMG) activity in thigh muscles (biceps femoris) of the right leg, and trunk muscles (erector spinae) on the left side, precede EMG activity in the shoulder muscles (deltoids) of the right arm and the subsequent first indices of right arm movement. These anticipatory adjustments can be viewed as either separable from (e.g., Massion, 1992; Turvey, Shaw & Mace, 1978) or integral to (e.g., Aruin & Latash, 1995; Friedl et al., 1984) the focal movement. Contemporary understanding favors the latter view (Patron, Stapley, & Pozzo, 2005; Stapley, Pozzo, Cheron & Grishin, 1999). If anticipatory adjustments are integral to voluntary movements, such as a lexical decision, these anticipatory muscle activations may be influenced by the same cognitive factors that have been shown to influence key presses.
In the present experiment we inquired whether lexical decision would be manifest in these anticipatory adjustments. Specifically we asked whether the RT difference between the onsets of yes and no arm movements—the difference at the focal movement level—would be present beforehand in biceps femoris and erector spinae, and by how many milliseconds.
The backdrop for the experiment is the proposed expansion of mental chronometry by Abrams and Balota (1991) and Balota and Abrams (1995). In respect to lexical decision, they augmented traditional inquiry into the influence of lexical factors (e.g., word frequency) on when a lexical decision response is initiated with inquiry into the influence of those factors on how a lexical decision response is executed. For lexical decision expressed by moving a lever in one of two directions, Abrams and Balota (1991) found that word frequency not only expedites response initiation but also amplifies the speed and force of response execution. This finding was replicated and extended in subsequent experiments using non-identical response formats for yes and no decisions (Balota & Abrams, 1995). The extension was that response execution was found to be sensitive to a factor (stimulus degradation) presumed to affect early phases of word processing as well as a factor (word frequency) presumed to affect later phases. The sensitivity of the response kinetics to the two factors was time-dependent: 20 ms into execution, frequency affected primarily the force of response to nondegraded stimuli; 50 ms into execution, frequency affected primarily the force of response to degraded stimuli.
The implication of the above findings is that continuous sampling of behavior subsequent to response initiation can be informative about the cognitive processes that precede response initiation (Balota & Abrams, 1995). Our expectation is that the use of whole body responding in lexical decision and other cognitive tasks should enhance inquiry into the how of response execution, and facilitate understanding of cognitive processes, by providing measures of temporal evolution and organization (such as patterns of relative timing and relative magnitudes of muscular activity from postural to focal levels). Such measures would increase the opportunities for detecting and identifying the varied forms of cognitive influences on response execution in reaction time paradigms. The present experiment is a first step toward implementing a whole body reaction-time methodology, one that might extend beyond lexical decision to benefit inquiry into mental chronometry and brain-body coordination.
Method
Participants
Ten males and one female were recruited from the University of Connecticut and Haskins Laboratories. All participants were right handed native English speakers with normal or corrected to normal vision. None of the participants reported any muscular disorders. All participants reported having normal reading competency. All gave their consent in accordance with the regulations of the University of Connecticut’s internal review board’s or Yale University’s internal review board’s for studies with human participants.
Materials and apparatus
Seventy-two words (middle and high frequency nonhomographs) were drawn from Rubinstein, Lewis, and Rubinstein’s (1971a) word list and 72 nonwords (pronounceable letter-strings that did not form legitimate English words) were drawn from Rubinstein, Lewis, and Rubinstein’s (1971b) nonword list. The average word frequency count for the word stimuli was 29.2±30 words per million (Kucera & Francis, 1967) (29.9±29 words per million according to CELEX). The average orthographic neighborhood size for words and nonwords was 4.5 words (±4.26) and 4.7 words (±3.32) respectively. All words and nonwords were 4-5 letters in length. There were no significant word-nonword differences in any of the aforementioned variables.
EMG activity was recorded using an 8-channel Dylsys Bagnoli desktop system (Delsys Inc., Boston, MA). The Bagnoli desktop system gathered EMG readings from six dual contact surface sensors (DE-2.1, Delsys Inc, Boston, MA) and movement data using two fiber optic goniometer sensors (Shape Sensor S700, Measurand Inc. NB, Canada).
Procedure
Participants were initially prepared for the experiment by locating target muscles and attaching the sensors. EMG sensors were securely attached to prepared skin near the mid-point of the right and left anterior deltoid (shoulder), right and left biceps femoris (thighs), and right and left paraspinal (primary erector spinae) muscles (lower back) at the level of the iliac crest. A reference surface-electrode was attached to the mid-point of either the left or right patella (knee cap). The ends of the goniometers (angle measurers) were positioned and attached to the upper arm and the shoulder blade of each arm so that the fiber optic cables formed a 90 deg angle when the arm was lifted.
After all the sensors had been attached and their signals tested, the nature of the lexical decision task was explained to the participants. Participants were asked to stand approximately 1 m in front of a computer screen set 1.75 m above the floor with their arms by their sides. Participants were instructed to indicate whether the letter-string on the screen was a word or not by elevating the appropriate arm forward to the horizontal as rapidly as possible. In order to avoid the potential confound between lexicality and handedness, a yes response (“the letter string is a word”) required movement of the right arm for six participants, and movement of the left arm for five participants.
The experimenter initiated the start of each lexical decision trial by pressing the space bar on a keyboard. Each trial began with a blank black screen for 500 ms. Onset of the screen initiated the recording of EMG and movement activity that continued for 7 sec. A fixation stimulus (a white circle) was displayed against a black background for 100 ms followed by another blank screen displayed for a variable amount of time (400-3400 ms). This variable blank screen was followed by the letter string which appeared centered on the screen for 1000 ms. The white letters against a black background subtended a visual angle roughly 0.48 deg horizontally and 0.97 deg vertically. The room was darkened to increase the contrast between the letters and the background. The lexical decision task began with ten practice trials (5 words and 5 nonwords not used in the experiment proper) followed immediately by 134 test trials.
EMG onset measurement
We used a procedure of determining onset EMG from an ensemble average. On each trial i of a given combination of responding arm (left or right) and lexicality (word or nonword) we acquired for each muscle of each participant a time series of EMG voltages, xi(t), and a goniometric shoulder angle measurement for the responding arm, θi(t). The beginning of the shoulder movement onset was used to define an anchor point such that aligned each xi(t) to a common origin. Movement onset was defined to be the first time at which (arm acceleration) exceeded 3 deg/s after the latest time at , but before the time of peak angle, max θi.
Within each condition for each participant, we computed an ensemble average of xi(t) for each muscle according to
| (1) |
where is the time-average of xi(t). We also computed a cumulative sum y(t) defined as
| (2) |
Identifying τ, the onset of ensemble muscle activation, required separating focal from background activation. We took background activation to be relatively constant in the ensemble average and removed its effect by subtracting the linear trend from y(t), that is,
| (3) |
where b0 and b1 are linear regression coefficients. Local minima of z(t) identified those places where cumulative activation began to rise faster than background activation. Accordingly our criterion for the activation onset τ was defined as
| (4) |
This quantity provides an average activation onset time relative to focal arm movement. Onsets were computed per muscle for each participant for all experimental conditions. These onsets were used to define lexical decision latencies at the muscle level.
Results
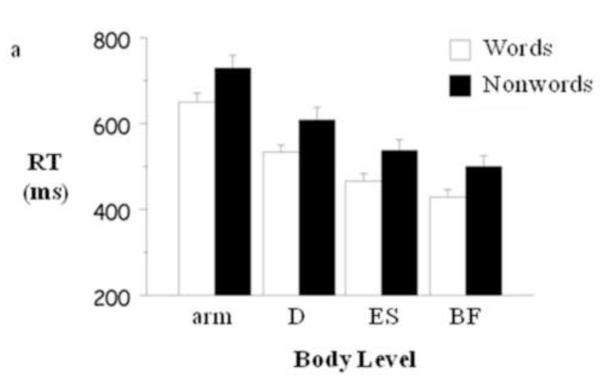
Figure 1a reveals that lexical decision was manifest at all measurement sites. The figure presents the lexical decision latencies to words and nonwords for the arm and the three muscles averaged over assignment of arm to yes and no responding. Mean onset latency decreased systematically across arm motion (689.05±92.20 ms), shoulder (568.91±89.98 ms), trunk (500.53±83.55 ms), and thigh (464.96±76.51 ms), F(3, 30) = 41.62, p < .0001, partial η2 = .81. Further, whereas word RT was uniformly less than nonword RT (means of 518.53±105.50 ms and 593.20±123.84 ms, respectively), F(1, 10) = 34.23, p < .001, partial η2 = .77, the magnitude of this difference was relatively constant from thigh (73 ms), to trunk (71 ms), to shoulder (75 ms), to arm motion (79 ms), F < 1.1
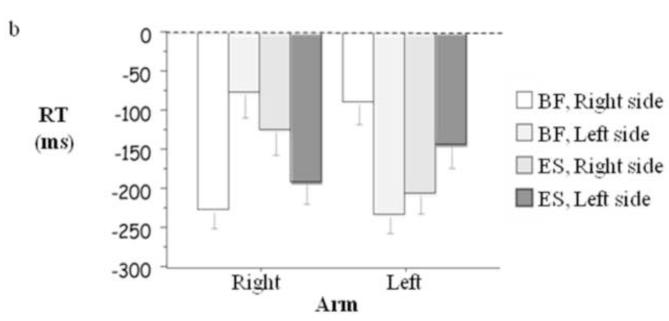
Figure 1.
(a) Word latencies and nonword latencies for arm movement, deltoid (D), erector spinae (ES), and biceps femoris (BF) averaged over responding arm. (b) Latencies of ES and BF relative to arm movement onset as a function of responding arm and side of body averaged over word and nonword decisions.
Figure 1b presents RT as a function of the responding arm averaged over yes and no responses. It reveals, in accord with the standard finding (e.g., Belenkii et al., 1967), that the pattern of EMG latencies across left and right biceps femoris, and left and right erector spinae, was specific to the responding arm, F(1, 10) = 84.34, p < .0001, partial η2 = .89.
Discussion
We found that the RT difference between the onsets of yes and no arm movements was invariant over body segments and, relative to stimulus onset, present in thigh, trunk, shoulder muscles, and arm movement, at 465, 501, 569 and 689 ms, respectively. We also found, as expected, that the patterning of onsets of thigh and trunk EMG activity was specific to the responding arm.2
Our findings warrant the speculation that the behavioral manifestation of the cognitive differentiation of word and nonword may be available at the earliest behavioral measure obtainable.3 As noted above, Braun, Jacobs, Hahne et al. (2006) found evidence for the resolution of the yes decision in the measure of event-related potentials at approximately 350 ms post-stimulus. Their average word response latency was 659 ms. In the present research we found evidence for the resolution of the yes decision in the measure of biceps femoris activity at approximately 429 ms post-stimulus for an average word response latency of 649 ms (see Figure 1a). On the assumption that the temporal indices of Braun, Jacobs, Hahne et al. (2006) and those of present experiment are comparable, one could infer that the lexical decision response was initiated minimally within 80 ms of the neural resolution of a letter string’s status as a word. In practical terms, the assumption and inference suggest that using both event-related potentials and whole body RT in lexical decision experiments could shed light on the temporal dimension of the decision-response, brain-body relation.
The present research was intended as spadework for the use of whole body RT to probe the how as well as the when of response execution in cognitive tasks such as lexical decision. The lexical decision experiments of Abrams and Balota (1991) and Balota and Abrams (1995) used the word-nonword response latency difference as the anvil on which to apply manipulations that might be manifest in response execution in addition to, and differently from, their manifestation in response latency. In Abrams and Balota (1991) the execution phase was approximately 130 ms in duration. In the present whole-body case, with response latency defined by the first anticipatory postural adjustment (biceps femoris), the execution phase is considerably longer, of the order of 1100-1200 ms. It includes the subsequent anticipatory postural adjustments and the time to raise the arm to the horizontal and provides, thereby, a larger anvil on which to work (discern) the cognitive influences. Consonant with the key kinematic features of the focal response in the lexical decision experiments of Abrams and Balota (1991) and Balota and Abrams (1995), the angular displacement, velocity, and acceleration at 150 ms into raising the arm were all significantly greater (p < .001) for words than for nonwords. In sum, whole body lexical decision expands the temporal window and amplifies the behavioral opportunities for exploring the claim that in lexical decision and naming tasks “early operations can enable appropriate action systems before the central decisions are made” (Balota & Abrams, 1995, p.1289).
Research Highlights.
• Presents a novel analysis of a fundamental information-processing reaction time task—Lexica Decision Task
• Extends the reconceptualization of the lexical decision task initiated by Abrams and Balota in the early 90s
• The experimental method holds promise for developing parallel mental and body chronometry.
Acknowledgments
Preparation of this manuscript was supported by National Institute of Child Health and Human Development Grant HD-01994 to the Haskins Laboratories.
Footnote
Analyses of the log transformed RT data repeated the main effects of lexicality, F(1, 10) = 39.43, p < .001, partial η2 = .798, and measurement site, F(3, 30) = 45.08, p < .001, partial η2 = .818.
Beyond involvement in body posture, anticipation is evident in muscles of the arm for seated finger tapping movements (akin to the key presses that are commonplace in lexical decision research) (Caronni & Cavallari, 2009). Surprisingly, the latter research reveals that several upper-limb muscles must coordinate as a postural chain to prohibit destabilizing effects of the interaction torques generated by the (seemingly) gentle, low inertia, finger tap. Of additional note, vocalizations of yes and no must be preceded by specific respiratory and laryngeal adjustments (e.g., Sawashima & Hirose, 1983), implying that early evidence of lexical decision ought to be found in verbal as well as nonverbal responses.
EMG is the obvious example of such a measure, but non-traditional measures can be considered such as ultrasound scanner and automated image analysis to record muscular changes at the scale of 10 μm (Loram, Maganaris & Lakie, 2005).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Miguel A. Moreno, Texas A & M University-Corpus Christi, and Haskins Laboratories
Nigel Stepp, Center for the Ecological Study of Perception and Action, University of Connecticut.
M. T. Turvey, Haskins Laboratories
References
- Abrams RA, Balota DA. Mental chronometry: Beyond reaction time. Psychological Science. 1991;2:153–157. [Google Scholar]
- Aruin AS, Latash ML. The role of motor action in anticipatory postural adjustments studied with self-induced and externally triggered perturbations. Experimental Brain Research. 1995;106:291–300. doi: 10.1007/BF00241125. [DOI] [PubMed] [Google Scholar]
- Balota DA, Abrams RA. Mental chronometry: Beyond onset latencies in the lexical decision task. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1995;21:1289–1302. doi: 10.1037//0278-7393.21.5.1289. [DOI] [PubMed] [Google Scholar]
- Belenkii YY, Gurfinkel V, Paltsev YI. Elements of control of voluntary movements. Biofizika. 1967;12:135–141. [PubMed] [Google Scholar]
- Braun M, Jacobs AM, Hahne A, Ricker B, Hofmann M, Hutzler F. Model-generated lexical activity predicts graded ERP amplitudes in lexical decision. Brain Research. 2006;1073-1074:431–439. doi: 10.1016/j.brainres.2005.12.078. [DOI] [PubMed] [Google Scholar]
- Caronni A, Cavallari P. Anticipatory postural adjustments stabilise the whole upper-limb prior to a gentle index finger tap. Experimental Brain Research. 2009;194:59–66. doi: 10.1007/s00221-008-1668-2. [DOI] [PubMed] [Google Scholar]
- Friedli WG, Hallett M, Simon SR. Postural adjustments associated with rapid voluntary arm movements: I. Electromyographic data. Journal of Neurology, Neurosurgery & Psychiatry. 1984;47:611–622. doi: 10.1136/jnnp.47.6.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kučera H, Francis WN. Computational analysis of present-day American English. Brown University Press; Providence, RI: 1967. [Google Scholar]
- Loram ID, Maganaris CN, Lakie M. Active, non-spring-like muscle movements in human postural sway: how might paradoxical changes in muscle length be produced? Journal of Physiology. 2005;564:281–293. doi: 10.1113/jphysiol.2004.073437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massion J. Movement, posture and equilibrium: interaction and coordination. Progress in Neurobiology. 1992;38:35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- Paltsev YI, Elner AM. Preparatory and compensatory period during voluntary movement in patients with involvement of the brain of different localization. Biofizika. 1967;12:142–147. [PubMed] [Google Scholar]
- Patron J, Stapley PJ, Pozzo T. Human whole-body reaching in normal gravity and microgravity reveals a strong temporal coordination between postural and focal task components. Experimental Brain Research. 2005;165:84–96. doi: 10.1007/s00221-005-2283-0. [DOI] [PubMed] [Google Scholar]
- Rubinstein H, Lewis SS, Rubinstein MA. Homographic entries in the internal lexicon: Effects of systematicity and relative frequency of meanings. Journal of Verbal Learning and Verbal Behavior. 1971a;10:57–62. [Google Scholar]
- Rubinstein H, Lewis SS, Rubinstein MA. Evidence for phonemic recoding in visual word recognition. Journal of Verbal Learning and Verbal Behavior. 1971b;10:645–657. [Google Scholar]
- Sawashima M, Hirose H. Laryngeal gestures in Speech Production. In: MacNeilage PF, editor. The Production of Speech. Springer-Verlag; New York: 1983. pp. 11–38. [Google Scholar]
- Stapley PJ, Pozzo T, Cheron G, Grishin A. Does the coordination between posture and movement during human whole-body reaching ensure center of mass stabilization? Experimental Brain Research. 1999;129:134–146. doi: 10.1007/s002210050944. [DOI] [PubMed] [Google Scholar]
- Turvey MT, Shaw RE, Mace W. Issues in the theory of action: Degrees of freedom, coordinative structures and coalitions. In: Requin J, editor. Attention and performance VII. Erlbaum; Hillsdale, NJ: 1978. pp. 557–595. [Google Scholar]