Fig. 2.
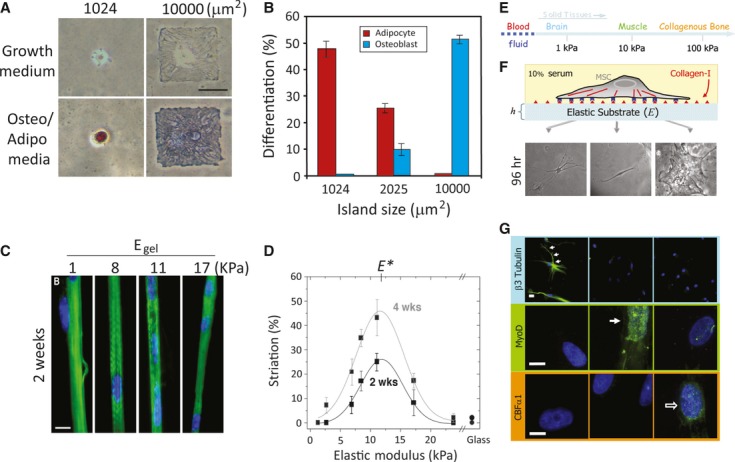
Mechanical stimulus–induced differentiation. (A) Cell shape drives mesenchymal stem cell (MSC) lineage commitment. Human (h)MSCs became bone only on large micropatterned islands, whereas adipogenesis occurred on small islands. (B) Quantitative results of MSC commitment on different-sized islands. Both A and B were reproduced from Ref. 58. (C) Myocytes cultured on collagen-coated polyacrylamide (PA) gels with various stiffness levels. Striated myotubes formed only on gels of intermediate stiffness. (D) Quantification results of optimal myotube formation on gels with different stiffness levels. Both C and D were reproduced from Ref. 80. (E) The elastic modulus of solid tissues. (F) The stiffness of the PA gel system can be modulated by changing the amount of the crosslinker. Cell adhesion to the gel can be controlled by covalent attachment of extracellular matrix (ECM) proteins (in this case, type 1 collagen). Human mesenchymal stem cells (hMSCs) seeded onto PA gels with different stiffness levels showed different morphologies. Cells were unspread with a branched morphology on soft substrate (0.1–1 kPa), had a bipolar morphology on intermediate stiffness (8–17 kPa) and had a polygonal morphology on stiff substrate (25–40 kPa) 96 hrs after seeding. (G) hMSCs differentiated into a neuronal lineage on soft substrate (0.1–1 kPa; as indicated by staining of βIII tubulin staining in cell branches); myogenic on intermediate stiffness (8–17 kPa; as indicated by MyoD staining of nuclei), and osteogenic on stiff substrate (as indicated by the punctuate CBFα1 staining of nuclei). E, F and G were reproduced from Ref. 82 (© 2004 Rockefeller University Press. Originally published in J. Cell Biol. 166:877–887).
