Abstract
Background
The addition of bevacizumab (BEV) to cytotoxic chemotherapy regimens (CTX) was believed to be effective; however, its magnitude of benefits is still controversial. So a meta-analysis and systematic review seems to be necessary.
Methods
PubMed and the Cochrane library were systematically searched. All relevant citations comparing CTX with/without BEV were considered for inclusion. Sensitivity and meta-regression analysis were performed to identify potential confounders. All pooled estimates were performed using a random-effects model. All statistical analyses were performed by StataSE 12.0.
Results
The search strategy identified 10 eligible random control trials (RCTs) (n=1366). In our pooled estimates, the additional benefits of BEV to CTX were identified in overall survival (OS) hazard ratio (HR, 0.76; 95% CI, 0.69 to 0.82) and progression-free survival (PFS) (HR, 0.56; 95% CI, 0.51 to 0.60), and prolonged survival duration were also identified for OS (18.2 vs. 16.3, p=0.0003) and PFS (8.9 vs. 6.5, p<0.001). Subgroup analyses stratified by CTX was also performed, evident benefits of additional BEV in OS and PFS can be identified in all subgroups, except for the CTX containing capecitabine in OS. Moreover, the increased rate of incidence was also identified in hypertension, thrombosis, proteinuria, gastrointestinal perforation, and fatigue.
Conclusion
BEV, acting as a targeted agent to CTX, its additional benefit to CTX is at the cost of increased toxicity.
Key words: bevacizumab, colorectal cancer, cytotoxic chemotherapy regimens, meta-analysis
Introduction
Colorectal cancer (CRC) is the third common and fourth leading cause of deaths among cancer sufferers throughout the world.1 Since intravenous Fluoropyrimidine therapy was first found to be efficacious for the treatment of metastatic CRC (mCRC), two other cytotoxic drugs (Irinotecan [IRI] and Oxaliplatin [OXA]) and targeted monoclonal antibodies (Bevacizumab (BEV), Cetuximab, and Panitumumab) had been gradually discovered over the last decades.2 OXA-based chemotherapy and 5-fluorouracil (FLU) plus Leucovorin (LEU, also known as folinic acid, acting as a biochemical modulator of FLU) based chemotherapy have become the standard treatment for mCRC.3–5 Moreover, Capecitabine (CAP) is an oral Fluoropyrimidine that has similar efficacy with the combination of FLU and LEU in the first-line treatment for mCRC.6–8
Acting as a humanized variant of anti-VEGF monoclonal antibody, BEV has been evaluated as an antiangiogenic cancer therapy in many tumor types.9 The primary mechanism of BEV is the inhibition of tumor growth rather than cytoreduction.10 It has antiangiogenic effect which could decrease local vascular density, and finally reduces the blood supply which is critical to the rapid growth of transplanted tumors.11 However, in addition to its direct antiangiogenic effect, BEV may also alter tumor vasculature and decrease the elevated interstitial pressure in tumor, such improves the delivery of chemotherapy.10,12,13 Additionally, BEV is well tolerated as a single agent, and also in combination with chemotherapy,10,14 but it does not have significant activity as monotherapy.15
However, with the gradually updating evaluation performed, the magnitude of additional benefits derived from BEV is still controversial. The present meta-analysis and systematic review has been performed with the purpose of assessing the feasibility and safety of BEV when adding to cytotoxic chemotherapy regimens (CTX) in the treatment of CRC.
Methods
Selection criteria
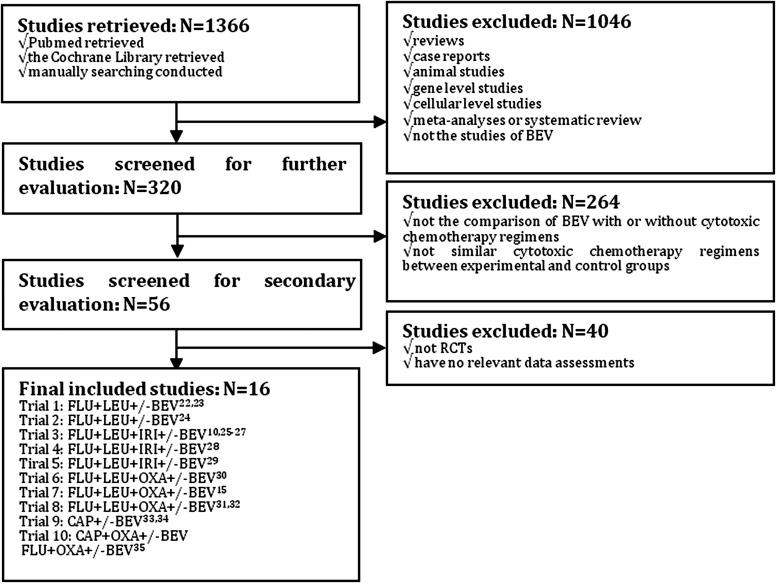
Studies were potentially eligible for inclusion in this meta-analysis if they involved a randomize comparison of CTX with/without additional targeted agent-BEV in the treatment of CRC patients (age >18), and CTX in both compared groups should not be confounded by additional chemotherapeutic, adjuvant agents or interventions. Prior surgical cancer therapy was permitted. Exclusions were considered if: abstract reports of RCTs presenting preliminary or interim data only, results of RCTs were reported in letter or editorials. Other reasons for exclusion were illustrated in Figure 1. Major selective criteria of patients, and details of chemotherapy regimens for each trial were shown in Table 1.
FIG. 1.
Flow chart of included trials.
Table 1.
Selected Characteristics of Included Randomized Controlled Trials
| |
|
Prior therapy (%) |
|
|
Quality assessment |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trials | Treatment allocation | AC | RT | SG | Selected patient inclusion criteria | Schedule of chemotherapy regimens | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Trial 1 (a) | FLU/LEU/BEV(5 mg/kg), n=35 | 14 | 14 | 80 | Histologically confirmed mCRC with metastases >1 cm; ECOG 0 or 1; life expectancy>3 months; age≥18; no prior AC, RT (other than fluoropyrimidines with FLU/LEU and/or levamisole>12 months before day 0) or SG within 28 days before day 0 | FLU, 500 mg/m2, IV; LEU, 500 mg/m2, IV; once weekly for 6 weeks, every 8 weeks, 6 cycles; BEV, 5 mg/kg, IV for trial 1 (a); every 2 weeks, for 48 weeks; BEV, 10 mg/kg, IV for trial 1 (b); every 2 weeks, for 48 weeks | U | U | L | L | L | U | H |
| FLU/LEU, n=36 | 21 | 15 | 85 | ||||||||||
| Trial 1 (b) | FLU/LEU/BEV(10 mg/kg), n=33 | 22 | 14 | 97 | |||||||||
| FLU/LEU, n=36 | |||||||||||||
| Trial 2 | FLU/LEU/BEV(5 mg/kg), n=104 | 19 | 15 | — | Histologically confirmed mCRC, previously untreated; not optimal candidates for first-line IRI containing therapy; without major surgery within 28 days; at least one of the following characteristics: age≥65; or ECOG 1 or 2; or serum albumin≤3.5 g/dL; or prior RT to abdomen or pelvis | FLU, 500 mg/m2, IV; LEU, 500 mg/m2, IV; once weekly for 6 weeks, every 8 weeks, 6 cycles; BEV, 5 mg/kg, IV; every 2 weeks, for 48 weeks | L | L | L | L | L | U | H |
| FLU/LEU/placebo, n=105 | 21 | 14 | — | ||||||||||
| Trial 3 | IFL/BEV(5 mg/kg), n=402 | 24 | 15 | — | Histologically confirmed mCRC; ECOG 0 or 1; age≥18; life expectancy>3 months; no prior AC (other than fluoropyrimidines with or without leucovorin or levamisole>12 months) or biologic therapy for metastatic disease; without major surgery within 28 days | IRI, 125 mg/m2, IV; FLU, 500 mg/m2, IV; LEU, 20 mg/m2, IV; once weekly for 4 weeks, every 6 weeks, 16 cycles; BEV, 5 mg/kg, IV; every 2 weeks, for 96 weeks | L | L | L | L | L | U | U |
| IFL/placebo, n=411 | 28 | 14 | — | ||||||||||
| Trial 4 | IFL/BEV(10 mg/kg), n=114 | — | — | 100 | Histologically confirmed stage IV CRC, including single or multiple metastases; prior surgery and the primary tumor was excised; ECOG 0–2; age≥18; without a second malignancy | IRI, 135 mg/m2, IV; FLU, 500 mg/m2, IV; LEU, 200 mg/m2, IV; on day 1, every 3 weeks, 8 cycles; BEV, 7.5 mg/kg, IV; on day 1, every 3 weeks, 8 cycles | U | U | U | U | L | U | U |
| IFL, n=108 | — | — | 100 | ||||||||||
| Trial 5 | IFL/BEV(5 mg/kg), n=139 | 50.4 | 12.2 | — | Histologically confirmed mCRC; age≥18; ECOG 0 or 1; no prior therapy for metastatic disease; life expectancy>3 months | IRI, 125 mg/m2, IV; FLU, 500 mg/m2, IV; LEU, 20 mg/m2, IV; once weekly for 4 weeks, every 6 weeks, 16 cycles; BEV, 5 mg/kg, IV; every 2 weeks, for 96 weeks | U | U | L | L | L | U | U |
| IFL, n=64 | 39.1 | 18.8 | — | ||||||||||
| Trial 6 | FOLFOX4/BEV(10 mg/kg), n=293 | 80a | 26a | 93a | Histologically confired advanced or metastatic adenocarcinoma of the colon and rectum prior treated with fluoropyrimidine- or IRI-based regimen and recovery from toxicities; ECOG 0–2; not prior treatment with oxaliplatin or bevacizumab; major surgery within 28 days | OXA, 85 mg/m2, IV, on day 1; FLU, 400 mg/m2, IV, on day 1, and 600 mg/m2, IV, on day 2, over 22 hours; LEU, 10 mg/m2, IV, on day 1 and day 2; every 2 weeks; BEV, 10 mg/kg, IV; on day 1, every 2 weeks | U | U | L | L | L | U | U |
| FOLFOX4, n=292 | 80a | 26a | 93a | ||||||||||
| Trial 7 | FOLFOX4/BEV(10 mg/kg), n=286 | — | 25.9 | — | Histologically confirmed advanced or metastatic CRC; prior use of oxaliplatin or bevacizumab was not permitted; no major surgery within 28 days or RT within 14 days | OXA, 85 mg/m2, IV, on day 1; FLU, 400 mg/m2, IV, on day 1, and 600 mg/m2, IV, on day 2, over 22 hours; LEU, 10 mg/m2, IV, on day 1 and day 2; every 2 weeks; BEV, 10 mg/kg, IV; on day 1, every 2 weeks | U | U | L | L | L | U | U |
| FOLFOX4, n=291 | — | 24.7 | — | ||||||||||
| Trial 8 | FOLFOX6/BEV(5 mg/kg), n=1326 | — | — | 100 | Patients with stage II or III colorectal adenocarcinoma; ECOG 0 or 1; within 29 to 50 days after surgical removal of the primary tumor | OXA, 85 mg/m2, IV, on day 1; FLU, 400 mg/m2, IV, on day 1, and 2400 mg/m2, IV, over 46 hours; LEU, 400 mg/m2, IV, on day 1; every 2 weeks; BEV, 5 mg/kg, IV; on day 1, every 2 weeks | L | L | L | L | L | U | H |
| FOLFOX6, n=1321 | — | — | 100 | ||||||||||
| Trial 9 | CAP/BEV(7.5 mg/kg), n=157 | 28 | 15 | 81 | Histologic diagnosis of colorectal adenocarcinoma; ECOG≤2; life expectancy of at least 12 weeks; age≥18; without major surgical procedure within 28 days | CAP, 1250 mg/m2, oral, On day 1 to 14, twice daily, every 3 weeks; BEV, 7.5 mg/kg, IV; on day 1, every 3 weeks | U | U | L | L | L | U | U |
| CAP, n=156 | 22 | 12 | 79 | ||||||||||
| Trial 10 (a) | XELOX/BEV(7.5 mg/kg), n=350 | No more than 25% | Histologically confirmed mCRC, ECOG≤1; age≥18; life expectancy>3 months; no prior treatment with oxaliplatin or bevacizumab; RT or SG≥4 weeks | CAP, 1000 mg/m2, oral, OXA, 130 mg/m2, IV, On day 1 followed by CAP, On day 1 to 14, twice daily, every 3 weeks; for trial 10 (a); OXA, 85 mg/m2, IV, on day 1; FLU, 400 mg/m2, IV, on day 1, and 600 mg/m2, IV, on day 2, over 22 hours; LEU, 10 mg/m2, IV, on day 1 and day 2; every 2 weeks; for trial 10 (b); BEV, 5 mg/kg, IV; on day 1, every 3 weeks | L | L | U | U | L | U | H | ||
| XELOX/placebo, n=350 | |||||||||||||
| Trial 10 (b) | FOLFOX4/BEV(5 mg/kg), n=350 | ||||||||||||
| FOLFOX4/placebo, n=351 | |||||||||||||
an approximate estimate; 1, random sequence generation; 2, allocation concealment; 3, blinding of participants and personnel; 4, blinding of outcome assessment; 5, incomplete outcome data; 6, selective reporting; 7, other bias.
L, indicate low risk of bias; H, indicate high risk of bias; U, indicate uncertain risk of bias; IV, intravenous injection; FLU, 5-fluorouracil; LEU, Leucovorin; IFL, bolus 5-fluorouracil/folinic acid/irinotecan; IRI, Irinotecan; BEV, bevacizumab; CAP, Capecitabine; CRC, colorectal cancer; mCRC, metastatic colorectal cancer; ECOG, Eastern Cooperative Oncology Group; AC, adjuvant chemotherapy; RT, radiation chemotherapy; SG, surgery; OXA, oxaliplatin.
Identification of trials
Deadline for trials publication and/or presentation was March, 2012. Updates of RCTs were systematically searched through PubMed (www.ncbi.nlm.nih.gov/pubmed/), and the Cochrane library (www.thecochranelibrary.com/view/0/index.html). Manually searching of related reference lists of identified trials and bibliographies of relevant books and review articles was also performed to identify any articles missed by initial search or any possible unpublished data. The keywords “BEV,” “Avastin,” “colon,” “rectum,” “colorectum,” “carcinoma,” “neoplasma,” “tumor,” “cancer,” were retrieved in a multipurpose combination. The searching strategy applied to PubMed is listed as below (any keyword containing multiple forms, including its noun, adjective, or any other form could be replaced by *):
#1 colon*
#2 rect*
#3 colorect*
#4 ((#1) OR #2) OR #3
#5 carcinoma
#6 neoplasm*
#7 tumor
#8 cancer
#9 (((#5) OR #6) OR #7) OR #8
#10 bevacizumab
#11 Avastin
#12 (#10) OR #11
#13 ((#4) AND #9) AND #12
Appropriate modifications were made and identical search was performed through the Cochrane library during the same period, but did not identify any additional articles. Researches were also performed so that both completed and current trials could be identified. All titles and abstracts identified by search strategies were assessed for relevance. Full papers were obtained if potential relevance could be confirmed or uncertainty existed. Additionally, no language or date limitations were imposed. Unpublished trials were sought through electronic searches, but none could be identified. The literature search, inclusion and exclusion criteria establishment, identification of relevant articles were all performed and verified by both of the two investigators (D.Z., Y.L.W.) independently. Any uncertainty or discrepancies about the eligibility of a trial or particular treatment groups within trials were resolved by discussion with the involvement of a third investigator (C.L.), and final consensus was made.
Data collection and quality assessment
The data presenting the most comprehensive results comparing CTX with/without additional targeted agent-BEV was extracted into a form, which was made by one investigator and modified by other investigators. For studies that had more than one published reports, each eligible outcome was collected into the identical trials, then the outcome from most recent reports was used where there was a possibility of overlapping data. The primary outcome measure was overall survival (OS), which was defined as the time from randomization until death by any cause. Data for survivors were censored on the date of last follow-up. The secondary outcome was progression-free survival (PFS), which was defined as the time from randomization until progression or death by any cause. Data for survivors without progression were censored on the date of last follow-up. Trials presenting OS and/or PFS were considered for inclusion. Overall response rate (ORR) and several adverse events were also taken into consideration.
Quality assessments for eligible trials were evaluated when referring to the Cochrane collaboration's tool for assessing risk of bias within the Cochrane handbook 5.1.0 (available on www.cochrane-handbook.org/), and performed by extracting key methodological characteristics from published trials, including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. Details for eligible selected trials are shown in Table 1.
Analysis and statistics
To investigate the targeted adjuvant effects of BEV when adding to CTX, all results were then combined to give overall hazard ratios (HRs, for OS and PFS) or odds ratios (ORs, for ORR, or other adverse events) for all of the chemotherapy regimens. Subgroup analyses were also done for each specified category basing on the CTX, for example, FLU+LEU, IFL, FOLFOX and CAP-based chemotherapy, to further evaluate the additional benefits of BEV to each specified CTX.
We analyzed data by using a random-effects model due to the anticipated clinical heterogeneity across the included trials to provide more conservative estimates. Statistical heterogeneity was evaluated using the X2 test, and the I2 describing the percentage of total variance across trials due to heterogeneity rather than chance. A probability level for the X2 statistic ≤10% (p≤0.10) or an I2 ≥50% were considered indication of statistical heterogeneity. The possible existence of publication bias was explored by funnel plots through the trim and fill method.16 The recorded heterogeneity and presenting funnel asymmetry was identified by performing subgroup analyses with matching for CTX, and subsequently sensitivity analyses. AS for one or more study-level variables explaining the heterogeneity of related regimens were then explored in meta-regression models for all studies combined.17,18 In addition to subgroups based on CTX, we took sample size of each individual trial, dosage of BEV, percentage of surgery before chemotherapy as confounding factors.
Survival curves for OS and PFS are presented as simple (non-stratified) Kaplan-Meier curves, which were performed by combining relevant data extracted from the original Kaplan-Meier curves.19–21 Engauge Digitizer 4.1 (available on http://digitizer.sourceforge.net/) was applied to extracting the data from the curves. Log-rank test was used to identify the differences in OS and PFS between CTX with or without BEV.
All statistical analyses were done with StataSE 12.0.
Results
Up to March, 2012, 16 reports10,15,22–35 compared the combination of CTX with/without targeted agents-BEV. Several data were reused within several reports separately, for example, trial 1, trial 3, trial 8 and trial 9, which were described in Figure 1. The details of extracting eligible citations were also described in this Figure, with 7,127 people having CRC involved into final meta-analyses. Two trials22,23 underwent FLU+LEU based chemotherapy, with 349 patients involved; 3 trials10,25–29 underwent IFL-based chemotherapy, with 1,249 patients involved; 3 trials15,30–32 underwent FOLFOX based chemotherapy, with 3,834 patients involved; 1 trial underwent CAP based33,34 chemotherapy, with 313 patients involved; 1 trial35 underwent both CAP+OXA based and FOLFOX based chemotherapy, with 700 patients involved, respectively. Seven trials10,15,22–27,30–32 were completed in USA, the other three trials were completed in Greece,28 China29 and Australia,33,34 respectively.
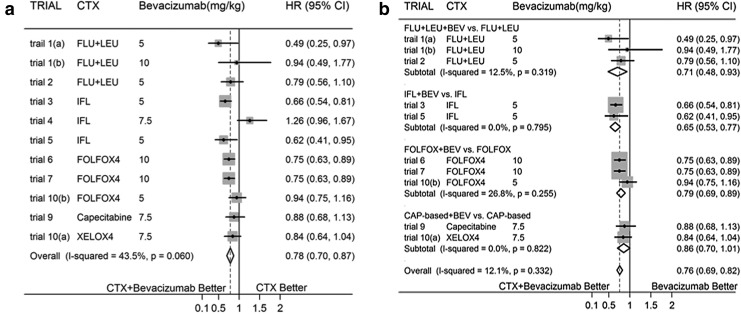
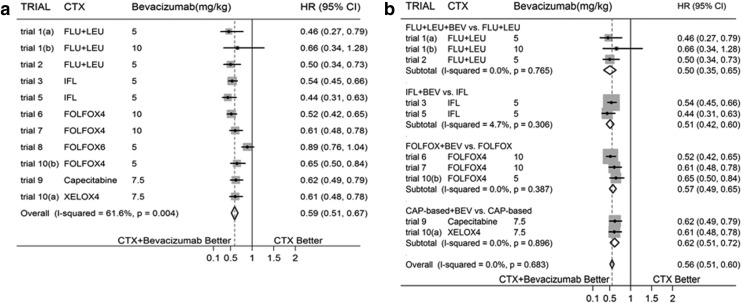
In meta-analyses done for OS and PFS when comparing targeted agent-BEV added to CTX versus CTX alone in the treatment of CRC, BEV is associated with evidently decreased HR [Fig. 2(a) showed overall effect for OS and Fig. 3(a) showed overall effect for PFS). And significant heterogeneity between trials could be identified (I2=43.5%, p=0.060 for OS; I2=61.6%, p=0.004 for PFS]. The results for both outcomes remained much the same when the above analyses were repeated with fixed-effects models.
FIG. 2.
(a) Overall Effect for Overall Survival (OS) (b) Subtotal Effect for OS.
FIG. 3.
(a) Overall Effect for Progression-Free Survival (PFS) (b) Subtotal Effect for PFS.
There were evidence of funnel-plot asymmetry for both outcomes about OS (p=0.020) and PFS (p=0.001). Subgroup analyses were done basing on different CTX, significantly decreased HRs for subtotal effects in OS were identified in the regimens about FLU+LEU based and FOLFOX based chemotherapies with/without BEV, with no evidence of statistical heterogeneity; while no significant differences were identified between CAP based chemotherapy with/without BEV (with no heterogeneity was identified) and IFL based chemotherapy with/without BEV, but with evidence of statistical heterogeneity (I2=80.1%, p=0.007) in IFL group. Sensitivity analyses were performed, and statistical heterogeneity disappeared after eliminating trial 4,28 which led to funnel-plot asymmetry. Additionally, subgroup analyses for PFS were done basing on similar CTX, with evident decreased HRs for all of the subgroups; but significant heterogeneity was found in FOLFOX group (I2=81.9%, q=0.001). Sensitivity analyses were performed, and then statistical heterogeneity disappeared after eliminating trial 831,32 which led to funnel-plot asymmetry.
In meta-regression analyses for OS, the associations between additional targeted chemotherapeutic agent-BEV and HRs were not substantially altered by sample size of each individual trial, dose of BEV, and CTX. However, they did depend on the percentage of prior surgery for CRC (p=0.012). In multivariate meta-regression analyses, after adjusted for these variables, percentage of prior surgery and CTX could explain 100% of between-study variance (p=0.0184), but with a stronger association with percentage of prior surgery than CTX (p=0.009). As for meta-regression analyses for PFS, the associations between BEV and HRs were not substantially altered by dose of BEV, and CTX. However, they did depend on the sample size of each individual trial (p=0.001) and the percentage of prior surgery for CRC (p=0.001). Both of these two confounding factors could explain 100% of between-study variance.
Subgroup analyses were reconfirmed after eliminating trial 4 in OS and trial 8 in PFS which were attributable to evident between-study heterogeneity. All subgroup analyses for both OS and PFS identified significantly decreased HR, excepting for CAP based CTX for OS [Fig. 2(b) and Fig. 3(b)].
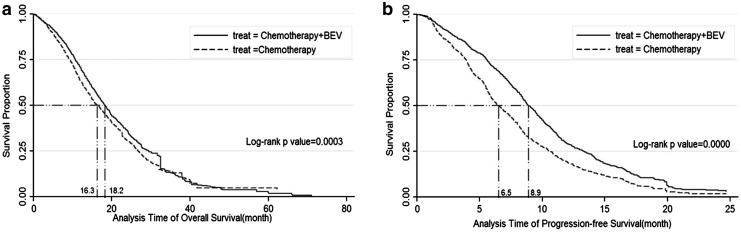
The OS and PFS endpoints were also described with Kaplan-Meier curves, with median survival duration prolonged approximate 1.9 months in OS (18.2 vs. 16.3, p=0.0003) and 2.4 months in PFS (8.9 vs. 6.5, p=0.0000), which were described in Figure 4(a) and Figure 4(b).
FIG. 4.
(a) Kaplan-Meier Survival Estimate for OS (b) Kaplan-Meier Survival Estimate for PFS.
Pooled estimates of ORs for ORR and several common grade 3 or 4 adverse events were also performed, and shown in Table 2. BEV tended to be associated with significantly higher OR when added to CTX (OR, 1.79; 95% CI, 1.28–2.50).
Table 2.
ORR and G3/4 Adverse Events
| |
ORR |
Any events |
Hypertension |
Thrombosis |
Bleeding |
Proteinuria |
GI perforation |
Diarrhea |
Leukopenia |
Fatigue |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trials | exp | con | exp | con | exp | con | exp | con | exp | con | exp | con | exp | con | exp | con | exp | con | exp | con |
| Trial 1 (a) | 14 | 6 | 26 | 19 | 3 | 0 | 5 | 1 | — | — | — | — | 0 | 0 | 10 | 13 | 2 | 1 | — | — |
| Trial 1 (b) | 8 | 6 | 25 | 19 | 8 | 0 | 2 | 1 | — | — | — | — | 3 | 0 | 10 | 13 | 1 | 1 | — | — |
| Trial 2 | 27 | 16 | 87 | 74 | 16 | 3 | — | — | 5 | 3 | 1 | 0 | 2 | 0 | 39 | 41 | 5 | 7 | — | — |
| Trial 3 | 180 | 143 | 334 | 294 | 43 | 9 | — | — | 12 | 10 | 3 | 3 | 6 | 0 | 127 | 98 | 145 | 123 | — | — |
| Trial 4 | 42 | 38 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Trial 5 | 49 | 11 | — | — | 4 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 26 | 21 | — | — | — | — |
| Trial 6 | 65 | 25 | — | — | 26 | 6 | — | — | 15 | 3 | — | — | — | — | 53 | 38 | — | — | 56 | 38 |
| Trial 7 | 65 | 25 | 216 | 174 | 18 | 5 | 10 | 7 | 10 | 1 | 2 | 0 | — | — | — | — | — | — | — | — |
| Trial 8 | — | — | — | — | 159 | 24 | 84 | 61 | 25 | 25 | 36 | 11 | 4 | 3 | 147 | 128 | — | — | 119 | 95 |
| Tiral 9 | 56 | 43 | — | — | 6 | 1 | 19 | 11 | 2 | 4 | 5 | 1 | 3 | 1 | 27 | 17 | — | — | 15 | 15 |
| Tiral 10 | 329 | 343 | — | — | 26 | 8 | 66 | 40 | 3 | 8 | 4 | 0 | 4 | 2 | — | — | — | — | — | — |
| Overall effect | ||||||||||||||||||||
| X2 test | 0.000 | 0.90 | 0.82 | 0.91 | 0.03 | 0.81 | 0.78 | 0.06 | 0.79 | 0.54 | ||||||||||
| OR (95%CI) | 1.79 (1.28,2.50) | 2.14 (1.71, 2.69) | 5.68 (4.26, 7.57) | 1.56 (1.23, 1.97) | 1.30 (0.68, 2.51) | 3.03 (1.73, 5.30) | 2.55 (1.10, 5.91) | 1.12 (0.88, 1.44) | 1.27 (0.95, 1.68) | 1.31 (1.05, 1.65) | ||||||||||
GI, gastrointestinal; exp, experimental group; con, control group; CI, confidence interval; ORR, objective response rate; OR, odds ratio.
As for study about the incidence of adverse events, the addition of BEV to CTX tended to be associated with significantly higher OR for any adverse events when compared with CTX alone (OR, 2.14; 95% CI, 1.71–2.69). The addition of BEV may also increase the incidence rate of grade 3 and 4 adverse events in hypertension, thrombosis, proteinuria, gastrointestinal perforation, and fatigue, with evident differences in ORs. But no evident between-group differences could be identified in the adverse events for bleeding, diarrhea, and leukopenia. Details were described in Table 2.
Discussion
Acting as a targeted antiangiogenic therapeutic agent for cancer, BEV provides little clinical benefits as a single agent,15 and its additional benefits to CTX varied among each individual trial in the treatment of CRC. This meta-analysis of individual trials identified that the addition of BEV to CTX was more effective comparing with CTX alone in improving the rate of survival in terms of OS and PFS, which may confirm the feasibility of adding targeted agents to CTX when treating CRC. And the median survival duration approximately prolonged 1.9 months in OS, and 2.4 months in PFS. The pooled estimate for ORR also identified improved response rate of BEV after adding CTX compared with CTX alone. It has a major prognostic effect on the survival of patients.36 The duration of BEV therapy is likely to be important, and treatment until disease progression may be necessary to maximize the clinical benefit derived from BEV therapy.35
However, the rate of any adverse events also increased, especially for hypertension, thrombosis, proteinuria, gastrointestinal perforation, and fatigue is grade 3/4. It was consistent with the controversy that the achievement of OS and PFS benefit with the addition of BEV to chemotherapy is at the cost of a significant increase in toxicity.37,38 Though grade 3/4 bleeding event was not evident in this meta-analysis, several reports had identified it.15,30,37 Moreover, Saltz et al.35 pointed out that neurotoxicity, gastrointestinal disorders, and hematologic events are the most common reasons for treatment discontinuation, which were attributable to CTX rather than BEV. Some trials had specified cerebral metastases, advanced atherosclerotic disease, or proteinuria as contraindications to the use of BEV.37
Additionally, frequent monitoring of blood pressure is necessary, and it is manageable with oral antihypertensives when hypertension occurs.37 The use of low-dose aspirin (≤325 mg/day) can also reduce the incidence of arterial thromboembolic events in high-risk patients treated with BEV,39 but no increased bleeding risk in patients receiving BEV and anticoagulation therapy.35
As aforementioned, the additional benefits of BEV were not consistent across trials. As for OS, no efficacy of additional BEV to CTX could be identified in several trials, including FLU+LEU,22,23 IFL,10 FOLFOX4,30 and CAP29,30 based chemotherapy regimens. However, after being stratified based on the CTX, the addition of BEV to FLU+LEU and FOLFOX based chemotherapy showed evident efficacy; its additional benefit was also evident in IFL-based chemotherapy after ignoring the trial10 leading greatly heterogeneity, suggesting that prior surgery treatment for CRC maybe a main confounding factor; as for CAP-based chemotherapy regimens, both trials29,30 identified no additional benefits of BEV. As for PFS, the additional benefits of BEV to CTX were identified in all trials except trial 1(b) and trial 8. The additional benefits were evident in all subgroups, after stratified basing on the CTX. And no alteration was identified after eliminating trial 8, which led to greatly heterogeneity mainly due to prior surgery treatment for CRC. Its largest sample size maybe also a confounding factor. Though adding BEV to CAP significantly improved PFS, but did not improve OS. It should be considered as a first line therapy option for patients with mCRC, especially when considering the benefits of controlling caner with limited adverse events, especially for those who are unfit for, or also do not require initial IRI or OXA.33
Some studies have also indicated that the benefit of BEV is independent of age, and that toxicity is not excessive either in older patient groups,33 or higher dose of BEV.10,22,24 However, cautions should be exercised in IRI contained regimens, since advanced age, prior pelvic radiation therapy, impaired performance status, and low serum albumin have all been reported to increase IRI-associated toxicities40–43; cautions should also be exercised in FOLFOX regimens, since the association of increasing age with excess toxicity,31,44 despite BEV was not associated with major additional toxicity except for a modest increase in rates of arterial thrombosis events in the older patient group.33
Additionally, predictive markers in relation to the treatment effects with/without BEV were also explored, and the survival benefit with the addition of BEV in the treatment of mCRC is independent of the status of biomarkers, such as K-ras, b-raf, and p-53.26–28,34 But D-dimer may represent a useful biomarker for patients treated with antiangiogenic agents, though strongly correlated with OS, but not PFS.23
In conclusion, BEV, acting as a targeted and antiangiogenic cancer therapeutic agent, is of efficacy and feasibility in adjuvant to CTX. However, the pros and cons should be considered since the generation of benefits is at the cost of toxicity. Further exploration should be considered to find out more directed prognosis predictors on the basis of additional efficacy of BEV to CTX, especially for each specific chemotherapy regimens.
Disclosure Statement
No competing financial interests exist.
References
- 1.Weitz J. Koch M. Debus J, et al. Colorectal cancer. Lancet. 2005;365:153. doi: 10.1016/S0140-6736(05)17706-X. [DOI] [PubMed] [Google Scholar]
- 2.Meyerhardt JA. Li L. Sanoff HK, et al. Effectiveness of bevacizumab with first-line combination chemotherapy for Medicare patients with stage IV colorectal cancer. J Clin Oncol. 2012;30:608. doi: 10.1200/JCO.2011.38.9650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hochster HS. Hart LL. Ramanathan RK, et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: Results of the TREE Study. J Clin Oncol. 2008;26:3523. doi: 10.1200/JCO.2007.15.4138. [DOI] [PubMed] [Google Scholar]
- 4.Thirion P. Michiels S. Pignon JP, et al. Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: An updated meta-analysis. J Clin Oncol. 2004;22:3766. doi: 10.1200/JCO.2004.03.104. [DOI] [PubMed] [Google Scholar]
- 5.Moertel CG. Chemotherapy for colorectal cancer. N Engl J Med. 1994;330:1136. doi: 10.1056/NEJM199404213301608. [DOI] [PubMed] [Google Scholar]
- 6.Van Cutsem E. Twelves C. Cassidy J, et al. Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: Results of a large phase III study. J Clin Oncol. 2001;19:4097. doi: 10.1200/JCO.2001.19.21.4097. [DOI] [PubMed] [Google Scholar]
- 7.Van Cutsem E. Hoff PM. Harper P, et al. Oral capecitabine vs intravenous 5-fluorouracil and leucovorin: Integrated efficacy data and novel analyses from two large, randomised, phase III trials. Br J Cancer. 2004;90:1190. doi: 10.1038/sj.bjc.6601676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoff PM. Ansari R. Batist G, et al. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: Results of a randomized phase III study. J Clin Oncol. 2001;19:2282. doi: 10.1200/JCO.2001.19.8.2282. [DOI] [PubMed] [Google Scholar]
- 9.Kabbinavar FF. Hambleton J. Mass RD, et al. Combined analysis of efficacy: The addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer. J Clin Oncol. 2005;23:3706. doi: 10.1200/JCO.2005.00.232. [DOI] [PubMed] [Google Scholar]
- 10.Hurwitz H. Fehrenbacher L. Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 11.Algire GH. Chalkley HW. Legallais FY, et al. Vascular reactions of normal and malignant tissue in vivo, I. Vascular reactions of mice to wounds and to normal and neoplastic transplants. J Natl Cancer Inst. 1945;6:73. [Google Scholar]
- 12.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: A new paradigm for combination therapy. Nat Med. 2001;7:987. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 13.Willett CG. Boucher Y. di Tomaso E, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margolin K. Gordon MS. Holmgren E, et al. Phase Ib trial of intravenous recombinant humanized monoclonal antibody to vascular endothelial growth factor in combination with chemotherapy in patients with advanced cancer: Pharmacologic and long-term safety data. J Clin Oncol. 2001;19:851. doi: 10.1200/JCO.2001.19.3.851. [DOI] [PubMed] [Google Scholar]
- 15.Giantonio BJ. Catalano PJ. Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 16.Peters JL. Sutton AJ. Jones DR, et al. Performance of the trim and fill method in the presence of publication bias and between-study heterogeneity. Stat Med. 2007;26:4544. doi: 10.1002/sim.2889. [DOI] [PubMed] [Google Scholar]
- 17.Thompson SG. Sharp SJ. Explaining heterogeneity in meta-analysis: A comparison of methods. Stat Med. 1999;18:2693. doi: 10.1002/(sici)1097-0258(19991030)18:20<2693::aid-sim235>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 18.Sterne JA. Juni P. Schulz KF, et al. Statistical methods for assessing the influence of study characteristics on treatment effects in ‘meta-epidemiological’ research. Stat Med. 2002;21:1513. doi: 10.1002/sim.1184. [DOI] [PubMed] [Google Scholar]
- 19.Parmar MK. Torri V. Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 20.Williamson PR. Smith CT. Hutton JL, et al. Aggregate data meta-analysis with time-to-event outcomes. Stat Med. 2002;21:3337. doi: 10.1002/sim.1303. [DOI] [PubMed] [Google Scholar]
- 21.Tierney JF. Stewart LA. Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kabbinavar F. Hurwitz HI. Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 23.Blackwell K. Hurwitz H. Lieberman G, et al. Circulating D-dimer levels are better predictors of overall survival and disease progression than carcinoembryonic antigen levels in patients with metastatic colorectal carcinoma. Cancer. 2004;101:77. doi: 10.1002/cncr.20336. [DOI] [PubMed] [Google Scholar]
- 24.Kabbinavar FF. Schulz J. McCleod M, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: Results of a randomized phase II trial. J Clin Oncol. 2005;23:3697. doi: 10.1200/JCO.2005.05.112. [DOI] [PubMed] [Google Scholar]
- 25.Choti MA. Bevacizumab in combination with irinotecan plus fluorouracil plus leucovorin chemotherapy prolongs survival but increases adverse events in people with metastatic colorectal cancer. Cancer Treat Rev. 2004;30:715. doi: 10.1016/j.ctrv.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Ince WL. Jubb AM. Holden SN, et al. Association of k-ras, b-raf, and p53 status with the treatment effect of bevacizumab. J Natl Cancer Inst. 2005;97:981. doi: 10.1093/jnci/dji174. [DOI] [PubMed] [Google Scholar]
- 27.Hurwitz HI. Yi J. Ince W, et al. The clinical benefit of bevacizumab in metastatic colorectal cancer is independent of K-ras mutation status: Analysis of a phase III study of bevacizumab with chemotherapy in previously untreated metastatic colorectal cancer. Oncologist. 2009;14:22. doi: 10.1634/theoncologist.2008-0213. [DOI] [PubMed] [Google Scholar]
- 28.Stathopoulos GP. Batziou C. Trafalis D, et al. Treatment of colorectal cancer with and without bevacizumab: A phase III study. Oncology. 2010;78:376. doi: 10.1159/000320520. [DOI] [PubMed] [Google Scholar]
- 29.Guan ZZ. Xu JM. Luo RC, et al. Efficacy and safety of bevacizumab plus chemotherapy in Chinese patients with metastatic colorectal cancer: A randomized phase III ARTIST trial. Chin J Cancer. 2011;30:682. doi: 10.5732/cjc.011.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen MH. Gootenberg J. Keegan P, et al. FDA drug approval summary: Bevacizumab plus FOLFOX4 as second-line treatment of colorectal cancer. Oncologist. 2007;12:356. doi: 10.1634/theoncologist.12-3-356. [DOI] [PubMed] [Google Scholar]
- 31.Allegra CJ. Yothers G. O'Connell MJ, et al. Initial safety report of NSABP C-08: A randomized phase III study of modified FOLFOX6 with or without bevacizumab for the adjuvant treatment of patients with stage II or III colon cancer. J Clin Oncol. 2009;27:3385. doi: 10.1200/JCO.2009.21.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allegra CJ. Yothers G. O'Connell MJ, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: Results of NSABP protocol C-08. J Clin Oncol. 2011;29:11. doi: 10.1200/JCO.2010.30.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tebbutt NC. Wilson K. Gebski VJ, et al. Capecitabine, bevacizumab, and mitomycin in first-line treatment of metastatic colorectal cancer: Results of the Australasian Gastrointestinal Trials Group Randomized Phase III MAX Study. J Clin Oncol. 2010;28:3191. doi: 10.1200/JCO.2009.27.7723. [DOI] [PubMed] [Google Scholar]
- 34.Price TJ. Hardingham JE. Lee CK, et al. Impact of KRAS and BRAF gene mutation status on outcomes from the phase III AGITG MAX trial of capecitabine alone or in combination with bevacizumab and mitomycin in advanced colorectal cancer. J Clin Oncol. 2011;29:2675. doi: 10.1200/JCO.2010.34.5520. [DOI] [PubMed] [Google Scholar]
- 35.Saltz LB. Clarke S. Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J Clin Oncol. 2008;26:2013. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 36.Buyse M. Thirion P. Carlson RW, et al. Relation between tumour response to first-line chemotherapy and survival in advanced colorectal cancer: A meta-analysis. Lancet. 2000;356:373. doi: 10.1016/s0140-6736(00)02528-9. [DOI] [PubMed] [Google Scholar]
- 37.Welch S. Spithoff K. Rumble RB, et al. Bevacizumab combined with chemotherapy for patients with advanced colorectal cancer: A systematic review. Ann Oncol. 2010;21:1152. doi: 10.1093/annonc/mdp533. [DOI] [PubMed] [Google Scholar]
- 38.Li S. Chi P. Optimizing the efficacy of first-line chemotherapy plus bevacizumab in metastatic colorectal cancer: Analysis of multiple methods. BioDrugs. 2011;25:43. doi: 10.2165/11584680-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 39.Cao Y. Tan A. Gao F, et al. A meta-analysis of randomized controlled trials comparing chemotherapy plus bevacizumab with chemotherapy alone in metastatic colorectal cancer. Int J Colorectal Dis. 2009;24:677. doi: 10.1007/s00384-009-0655-9. [DOI] [PubMed] [Google Scholar]
- 40.Bleiberg H. Cvitkovic E. Characterisation and clinical management of CPT-11 (irinotecan)-induced adverse events: The European perspective. Eur J Cancer. 1996;32A(Suppl 3):S18. doi: 10.1016/0959-8049(96)00293-6. [DOI] [PubMed] [Google Scholar]
- 41.Rougier P. Bugat R. CPT-11 in the treatment of colorectal cancer: Clinical efficacy and safety profile. Semin Oncol. 1996;23(1 Suppl 3):34. [PubMed] [Google Scholar]
- 42.Rougier P. Bugat R. Douillard JY, et al. Phase II study of irinotecan in the treatment of advanced colorectal cancer in chemotherapy-naive patients and patients pretreated with fluorouracil-based chemotherapy. J Clin Oncol. 1997;15:251. doi: 10.1200/JCO.1997.15.1.251. [DOI] [PubMed] [Google Scholar]
- 43.Rothenberg ML. Cox JV. DeVore RF, et al. A multicenter, phase II trial of weekly irinotecan (CPT-11) in patients with previously treated colorectal carcinoma. Cancer. 1999;85:786. [PubMed] [Google Scholar]
- 44.Goldberg RM. Tabah-Fisch I. Bleiberg H, et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol. 2006;24:4085. doi: 10.1200/JCO.2006.06.9039. [DOI] [PubMed] [Google Scholar]