Abstract
Despite efforts to improve surgical, radiologic, and chemotherapeutic strategies, the outcome of patients with glioblastoma (GBM) is still poor. Polo-like kinase 1 (PLK1) is a serine/threonine kinase that plays key roles in cell cycle control and has been associated with tumor growth and prognosis. Here, we aimed at testing the radiosensitizing effects of the PLK1 inhibitor BI 2536 on eight GBM cell lines. For cell cycle analysis, T98G, U251, U343 MG-a, LN319, SF188, U138 MG, and U87 MG cell lines were treated with 10, 50, or 100 nM of BI 2536 for 24 hours. In addition, cell cultures exposed to BI 2536 50 nM for 24 hours were irradiated with γ-rays from 60Cobalt source at final doses of 2, 4, and 6 Gy. Combinatorial effects were evaluated through proliferation and clonogenic capacity assays. Treatment with BI 2536 caused mitotic arrest after 24 hours, and increased apoptosis in GBM cells. Moreover, our results demonstrate that pretreatment with this drug sensitized six out of seven GBM cell lines to different doses of γ-irradiation as shown by decreased growth and abrogation of colony-formation capacity. Our data suggest that PLK1 blockage has a radiosensitizing effect on GBM, which could improve treatment strategies for this devastating tumor.
Key words: BI 2536, glioblastoma, mitotic arrest, polo-like kinase 1, radiotherapy
Introduction
Glioblastoma (GBM) is one of the most aggressive and common glial tumors, and it represents the main type of primary cancer of the central nervous system in adults. It is associated with high mortality, a median overall survival rate of 12.5 months in most cases, and a 2-year overall survival of 15.4%,1 being one of the hardest central nervous system tumors to be treated. The actual standard treatment for GBM combines tumor resection, chemotherapy, and radiotherapy.2
Gamma radiation is a locally effective and common treatment strategy used in cancer, mainly due to its high penetration that causes unrepairable DNA damage, leading to cell death, while limiting normal tissue toxicity.3 Nonetheless, the inherent radioresistance of GBM cells makes the treatment largely unsuccessful in most cases, and patients succumb to the disease. The inclusion of concurrent and adjuvant chemotherapy with the alkylating agent Temozolomide has only improved survival chance in a few more months.4 Thus, the search for novel agents with more effective antitumor activity is a high priority in the treatment of GBM.
Polo-like kinase 1 (PLK1) is a serine/threonine kinase that plays key roles in cell cycle progression and division5 and due to its function, this protein is found to be expressed in proliferative cells. PLK1 has a peak of expression in G2/M and acts as a regulator and promoter of the cell cycle: it activates the Cdk1/Cyclin B1 complex that promotes mitosis entrance, promotes chromosome maturation by recruitment of microtubules to the spindle pole bodies, and regulates the localization of centrosomal-associated proteins and the localization of Aurora B kinase for proper maturation and spindle assembly checkpoint. PLK1 also plays a key role in chromosome segregation, cytokinesis, and mitosis exit through phosphorylation and degradation of the anaphase promoter complex and its inhibitor.6
Altered PLK1 expression has been described in different neoplasias and correlated with prognosis.7 The inhibition of this kinase by different methods has been shown to cause cell cycle arrest and to increase apoptosis in several models.8 Moreover, depletion by siRNA has recently been reported to radiosensitize rectal and head-and-neck squamous carcinoma cells.9,10 Recently, BI 2536, an ATP-competitor dihydropteridinone, has been shown to efficiently inhibit the activity of PLK1.11 This drug has proved to be more than 1000 times specific for PLK1 than for other kinases.12 This compound has been tested in several tumor cells, albeit there are no reports on its radiosensitizing effects on GBM.
Methods
Cell culture
The adult human GBM cell lines T98G, U251, U343 MG-a, U138 MG, and U87 MG were purchased from the American Type Culture Collection, USA. LN319 cell line was kindly provided by Dr. Frank Furnari (Ludwig Institute for Cancer Research), and the pediatric SF188 cell line was kindly provided by Drs. Nada Jabado and Damien Faury (McGill University). Cells were cultured in HAM F10 (Gibco BRL, Life Technologies®) supplemented with 10% fetal bovine serum, penicillin (100 U/mL), and streptomycin (100 μg/mL) at 37°C in a humidified 5% CO2 incubator.
Drug and treatments
BI 2536 was purchased from Axon Medchem, and stock solutions were prepared in dimethyl sulfoxide (DMSO) according to the manufacturer's instructions and stored at −80°C. For experiments, cells were treated with nanomolar concentrations (10, 50, or 100 nM) in accordance to the literature.12 Vehicle alone was used as a control.
Cell irradiation
Cell cultures were irradiated with γ-rays from 60Cobalt at a dose rate of about 0.47 Gy/min, using a Gammatron S-80 equipment (Siemens Medical Systems, Inc.) at the Clinics Hospital of the Faculty of Medicine of Ribeirão Preto, University of São Paulo.
Dose enhancement ratios (DER) by BI 2536 were calculated using the following formula: DER=(surviving fraction at an indicated dose of radiation alone)/(surviving fraction at an indicated dose of radiation+BI 2536). DER ratio=1 suggests an additive radiation effect and DER >1, a supra-additive effect as against a sub-additive effect in the case of DER <1.13
Cell cycle analysis
For cell cycle analysis, 2×105 cells were seeded on 25 cm2 tissue culture flasks and treated with 10, 50, or 100 nM of BI 2536 for 24 hours. After treatment, cells were trypsinized, fixed in 70% ethanol, stained with propidium iodide, and analyzed on a Guava Personal Cell Analysis system (Guava Technologies) according to the standard protocol provided by the manufacturer. Percentages of cells in G0/G1, S, or G2/M phase were collected and processed using the GUAVA Cytosoft 4.2.1 version Software.
Apoptosis detection
3×104 cells were seeded on six-well plates and allowed to attach. After 24 hours, the medium was replaced, and the cells were treated with different concentrations of BI 2536 and cultured for an additional 24 hours. Briefly, the treated cells were trypsinized, centrifuged, then mixed with 300 μL of binding buffer, 5 μL Annexin V (BD Biosciences Pharmigen), and 50 μL propidium iodide (30 μg/mL), and incubated at 37°C for 15 minutes. Samples were then analyzed using the GUAVA Cytosoft 4.2.1 version Software. Five thousand events were analyzed per treatment, and cells were scored and categorized according to differential staining.
Measurement of cell growth by XTT cell proliferation assay
For proliferation assays, GBM cells were seeded in 25 cm2 culture flasks and treated with 50 nM BI 2536 for 24 hours, after which the medium was replaced and the cells were irradiated with 2, 4, and 6 Gy. After irradiation, the cells were trypsinized, seeded in 96-well flat-bottom plates, and incubated for 7 days. After that period, the culture medium was removed and replaced with medium containing 10 μL of XTT dye (3 mg/mL) (XTT II; Roche Molecular Biochemicals) in each well. The plates were incubated for 2 hours at 37°C, and the formazan product was measured at 450 nm by using an iMark microplate reader (Bio-Rad Laboratories®). Each experiment was performed in triplicate and was repeated in three sets of tests.
Colony formation assay
Clonogenic assays were performed according to Franken et al.14 After trypsinization, single cell suspensions of 500 cells were seeded in six-well plates and treated with BI 2536 at 50 nM concentrations for 24 hours; after that, the culture medium was removed and replaced with drug-free medium, and cells were treated with 2, 4, and 6 Gy of γ-radiation. Cell cultures were then incubated for 7 days, and the colonies were rinsed with PBS, fixed with methanol, and stained with Giemsa. Only colonies with >50 cells were counted. Assays were performed in triplicate.
The plating efficacy (PE) represents the percentage of cells seeded that grow into colonies under a specific culture condition of a given cell line, and it was calculated as the percentage of the number of colonies/number of cells seeded. The survival fraction (SF) was calculated by using the following formula: SF=(number of colonies formed for a specific treatment)/(number of cells seed)×(PE).14
Statistical analysis
Statistical analyses were performed by using the SigmaStat software (Jandel Scientific Company). One-way repeated-measures analysis of variance followed by the Holm–Sidak Pairwise Multiple Comparison was used to establish significant differences between groups. All tests were carried out for α=0.05.
Results
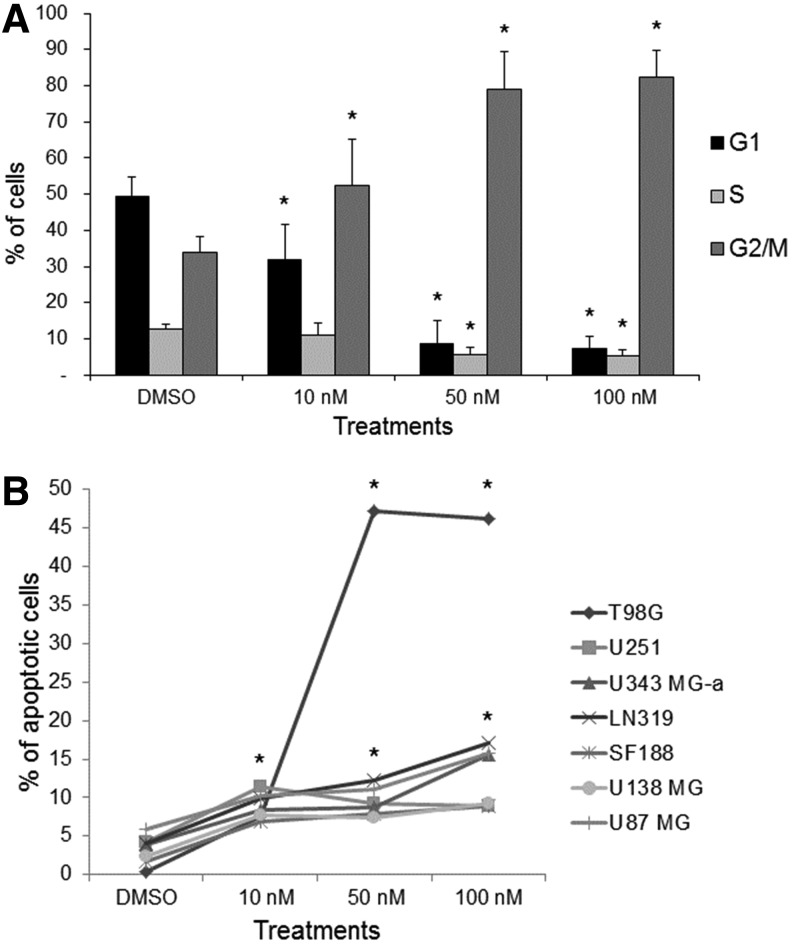
Cell cycle analysis showed that BI 2536 after 24 hours of treatment caused G2/M arrest in all GBM cell lines tested when compared with untreated controls (Fig. 1A) (see Table 1). Increased apoptosis was also observed after 24 hours of treatment with BI 2536 (Fig. 1B). Based on these results, the concentration of 50 nM was selected to be used for combinatorial experiments with ionizing radiation.
FIG. 1.
(A) BI 2536 treatments induced blockage in mitosis in glioblastoma (GBM) cells, with accumulation of dividing cells at G2/M phase after 24 hours of treatment in all GBM cell lines; (B) Increased apoptotic rates after treatment with BI 2536 in all GBM cells lines (data are expressed as the mean±SD of all cell lines). *p<0.05.
Table 1.
Cell Cycle Analysis of Glioblastoma Cell Lines Treated with BI 2536 for 24 Hours
| |
|
Cell line |
||||||
|---|---|---|---|---|---|---|---|---|
| T98G | U251 | U343 MG-a | LN319 | SF188 | U138 MG | U87 MG | ||
| G1 (%) | DMSO | 52.77±2.96 | 40.06±15.57 | 54.03±2.56 | 53.93±2.46 | 49.5±5.09 | 50.67±0.76 | 45.64±0.76 |
| BI 2536 10 nM | 38.21±7.54 | 29.63±3.41 | 20.40±3.58 | 33.66±2.86 | 18.41±2.37 | 44.72±1.84 | 37.68±4.65 | |
| BI 2536 50 nM | 10.15±5.65 | 22.22±15.61 | 4.57±0.80 | 6.29±1.76 | 5.43±2.88 | 4.6±0.49 | 6.8±5.23 | |
| BI 2536 100 nM | 10.25±5.16 | 10.77±8.48 | 11.17±10 | 3.99±0.26 | 5.65±0.62 | 5.21±2.02 | 3.36±0.26 | |
| S (%) | DMSO | 13.43±0.14 | 13.26±8.20 | 10.24±1.72 | 12.1±1.09 | 11.67±3.5 | 15.01±2.09 | 11.97±0.7 |
| BI 2536 10 nM | 12.50±0.22 | 11.38±6.1 | 8.94±0.63 | 7.04±1.10 | 8.67±5.2 | 17.08±0.57 | 11.2±0.18 | |
| BI 2536 50nM | 9.23±1.6 | 6.14±2.84 | 4.91±2.02 | 3.48±1.54 | 5.99±6.72 | 5.64±0.63 | 3.92±2.43 | |
| BI 2536 100 nM | 7.46±1.1 | 3.80±2.21 | 4.51±0.79 | 3.67±0.67 | 6.78±6.14 | 7.27±1.94 | 2.96±0.88 | |
| G2/M (%) | DMSO | 26.59±1.7 | 36.85±6.63 | 35.09±1.62 | 33.08±2.79 | 31.42±6.73 | 33.28±1.6 | 40.83±0.5 |
| BI 2536 10 nM | 36.03±5.58 | 55.19±8.53 | 69.58±3.62 | 58.96±2.33 | 61.59±1.55 | 35.84±1.16 | 48.91±5.38 | |
| BI 2536 50 nM | 64.69±8.16 | 66.02±22.84 | 89.41±3.16 | 90.00±3.00 | 78.59±5.52 | 81.93±6.12 | 83.57±7.59 | |
| BI 2536 100 nM | 70.78±4.15 | 82.58±9.30 | 81.53±9.42 | 91.81±0.24 | 78.1±4.52 | 80.65±5.9 | 91.64±1.05 | |
Percentages of cells in G1, S, and G2/M phases are expressed as mean±standard deviation of triplicates.
Bold values indicate p<0.05.
DMSO, dimethyl sulfoxide; SF, survival fraction.
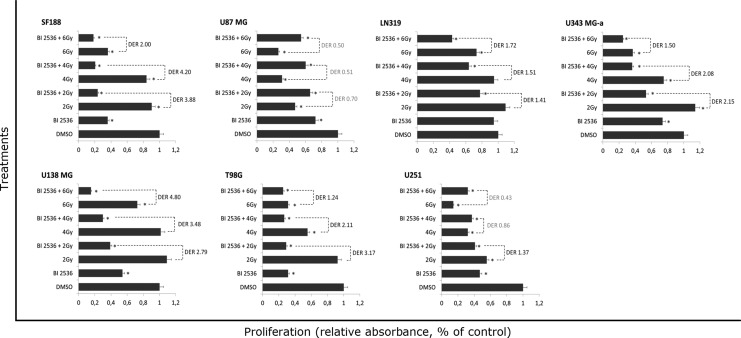
A significant increase in cell death for GBM cells was found after 24 hours of treatment with BI 2536 (Fig. 1B). Treatment of GBM cell lines with that concentration of BI 2536 for 24 hours also caused a significant inhibition in cell growth in all GBM cell lines used (except LN319) when compared with control (DMSO 0.1%) (p<0.05) (Fig. 2). Cell proliferation was reduced by 68.5% for T98G, 64% for SF188, 53% for U251, 47.5% for U138 MG, 27.8% for U87 MG, 26.4% for U343 MG-a, and 5% for LN319. When combined with 2, 4, and 6 Gy, results showed supra-additive effects for six out of seven GBM cell lines, being the strongest effect observed in the pediatric SF188 cell line (Fig. 2). On the other hand, U87 MG showed better results when treated with irradiation alone. However, proliferation was found to increase in some LN319, U138 MG, and U343 MG-a cell lines after treatment with 2 Gy; however, this increase was only significant for the U343 MG-a cell lines.
FIG. 2.
Reduced proliferation in GBM cell lines after treatment with BI 2536, γ-radiation, or combined treatment when compared with control (DMSO 0.2%) (*p<0.05) are shown for each GBM cell line as a percentage of absorbance relative to control. Pretreatment with BI 2536 50 nM significantly sensitized GBM cells to ionizing radiation. Dose enhancement ratios (DER)=1 suggests an additive radiation effect and DER >1, a supra-additive effect as against a sub-additive effect in the case of DER <1.
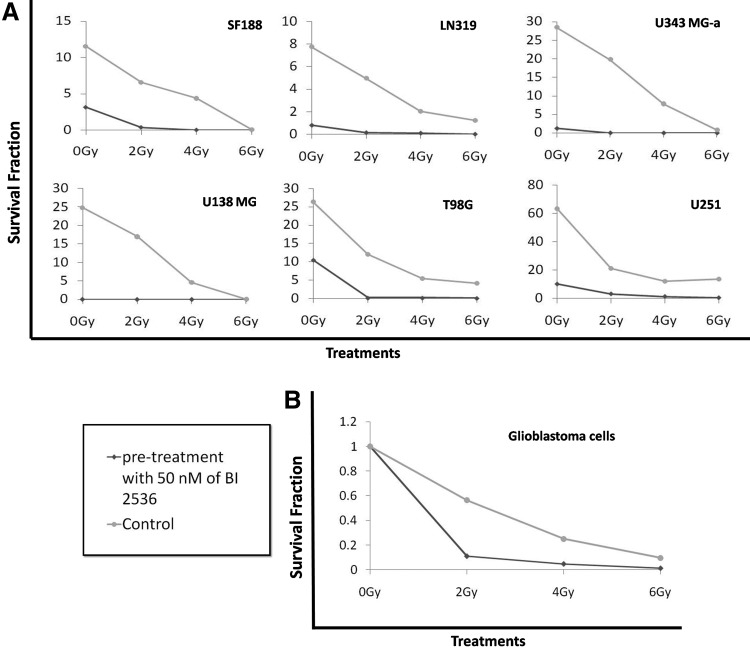
In addition, the analysis of the capacity of GBM cell lines to form colonies showed even more pronounced effects (see Table 3), being supra-additive (synergic) for all cell lines at all doses tested (see Table 2). In the case of U343 MG-a and U138 MG, the clonogenic capacity was entirely abrogated (Fig. 3A). In addition, to confirm the radiosensitizing effect of BI 2536, the clonogenic data were normalized to control, then compared with irradiated samples, and were presented as a mean of all GBM cell lines (Fig. 3B). Thus, PLK1 inhibition seems a good strategy to sensitize GBM cell lines to radiation.
Table 3.
Clonogenic Capacity of Glioblastoma Cell Lines Treated with BI 2536 and BI 2536+Irradiation for 24 Hours
| T98G | U251 | R286 | |
|---|---|---|---|
| DMSO | 26.3±2.21 | 63.34±8.08 | 103.04±6.42 |
| BI | 12.03±1.53 | 21.26±1.68 | 52.64±15.96 |
| 2 Gy | 5.5±0.68 | 12.25±1.88 | 21.08±2.33 |
| BI+2 Gy | 4.19±1.24 | 13.63±2.26 | 12.2±5.46 |
| 4 Gy | 10.49±1.42 | 10.11±5.26 | 22.95±4.55 |
| BI+4 Gy | 0.16±0.76 | 3.14±1.04 | 9.05±1.6 |
| 6 Gy | 0.16±0.1 | 1.30±0.37 | 1.98±1.24 |
| BI+6 Gy | 0.08±0.13 | 0.42±0.2 | 0.05±0.13 |
| U343 MG-a | LN319 | SF188 | U138 MG | |
|---|---|---|---|---|
| DMSO | 28.45±8.01 | 7.74±1.06 | 11.56±1.23 | 24.86±2.61 |
| BI | 19.82±5.15 | 4.96±1.75 | 6.59±1.31 | 17.03±7.5 |
| 2 Gy | 7.81±2.33 | 2.04±0.93 | 4.41±2.96 | 4.62±5.29 |
| BI+2 Gy | 0.84±0.62 | 1.23±0.8 | 0.11±1.16 | 0.11±3.04 |
| 4 Gy | 1.21±1.01 | 0.79±0.43 | 3.17±0.92 | 0.03±0.06 |
| BI+4 Gy | 0.03±0.07 | 0.15±0.14 | 0.38±0.48 | 0 |
| 6 Gy | 0 | 0.10±0.1 | 0.02±0.05 | 0 |
| BI+6 Gy | 0 | 0.01±0.03 | 0 | 0 |
Represent by the mean±standard deviation for each cell line.
Table 2.
Dose Enhancement Rates for each Glioblastoma Cell Line, Irradiation Versus BI 2536+Irradiation, Analyzed Through Clonogenic Assay
| 2 Gy | 4 Gy | 6 Gy | Cell line | |
|---|---|---|---|---|
| BI 2536 | 74.167 | 33.667 | 51.667 | T98G |
| 6.76 | 9.4194 | 32.5 | U251 | |
| 5.8156 | 10.649 | 228 | U343 MG-a | |
| 1872.7 | ∞ | ∞ | U343 | |
| 33.8 | 19.857 | 84 | LN319 | |
| 17.524 | 246 | ∞ | SF188 | |
| ∞ | ∞ | ∞ | U138 MG |
U87 cell line does not form colonies.
DER=1 suggests an additive radiation effect and DER >1, a supra-additive effect as against a sub-additive effect in the case of DER <1.
∞, no colonies were observed after treatment; DER, dose enhancement ratios.
FIG. 3.
(A) Reduced survival fraction (SF) in each GBM cell line after treatment with BI 2536, γ-radiation, or combined treatment when compared with control. (B) Relative SF in all GBM cell lines after treatment with BI 2536, γ-radiation, or combined treatment when compared with control (DMSO 0.2%) (*p<0.05).
Discussion
GBM represents a challenge in medicine because of its inherent chemo- and radioresistance, and also due to the complex and redundant activation of innumerous signaling pathways. The ongoing efforts to identify effective strategies for its treatment include combinations with drugs that target complementary or redundant pathways,2 among which we proposed and studied the combinatorial effects of PLK1 inhibition by BI 2536 with γ-radiation on seven GBM cell lines.
PLK1 is involved in cell cycle progression/division, controlling all stages of mitosis. Its deregulation has been shown to increase genomic instability, favoring cancer maintenance15; however, the heterogeneity and prevalence in its expression is directly associated with the proliferation rate of the cell.16 High levels of PLK1 have been described in different tumor cells, including GBM,17 and its expression level has even been correlated with prognosis.7 PLK1 inhibition by RNAi has been demonstrated to have an antitumoral effect, mainly causing mitotic arrest, aneuploidy, and apoptosis in several tumor types,18 reinforcing the role of this protein in tumor progression and maintenance.
BI 2536 is an ATP-competitor dihydropteridinone that has been shown to be a highly specific PLK1 inhibitor, causing blockage in mitosis and cell death.11 The pharmacological inhibition of PLK1 by BI 2536 is highly specific and has been proved to be well tolerated in intravenous dose regimens, efficiently crossed the brain-blood barrier, and inhibited tumor growth in vivo in several models,12 and due to this, it has already been tested in patients in whom it has been seen to be safe in phase I clinical trials.19,20 BI 2536 has been shown to have anticancer activities in different tumor cells,21 mainly due to the G2/M arrest. Moreover, PLK1 has been proved to be essential to DNA damage repair,22 and its depletion by BI 2536 could prevent the recovery from irradiation.
Here, we reported that PLK1 inhibition causes an accumulation of cells with a doubled DNA content and an increase in apoptosis rate in all the GBM cell lines tested. G2/M arrest after PLK1 inhibition has already been shown in different cell types, including fibroblastic, primary, and cancer cells.23–25 It is possible that after repeated efforts to divide, cells proceed toward death through apoptosis, which is the hallmark of PLK1 inhibition. Consistent with our findings, increased cell death rates have also been reported after PLK1 inhibition in pancreatic cancer cells,26 hepatocellular carcinomas cells,27 and squamous cell carcinoma cells,28 among others. These confirm the importance of this gene as a target for cancer treatment.
Compelling evidence has shown that combinatorial treatment with drugs that induce G2/M arrest could enhance the radiosensitivity of cells.29–33 In the present study, we demonstrate that PLK1 inhibition with BI 2536 causes mitotic arrest in GBM cell lines, and that pretreatment with this drug efficiently sensitizes cells to radiation by decreasing proliferation and self-renewal in all the cell lines tested. Decreased proliferation after treatment with BI 2536 alone has already been demonstrated in different tumors: cervix adenocarcinoma,24 leukemia,34 anaplastic thyroid carcinoma,35 melanomas,15 and osteosarcoma cells.25 Here, we also report that the combinatorial effect of BI 2536 with radiation was superior to treatment with the drug alone on cell proliferation, showing synergic effects. These data suggest the importance of PLK1 inhibition as a sensitizer to radiation in GBM cancer cells.
Comparatively, we also showed that clonogenic capacity of all GBM cell lines tested was significantly diminished after combined treatment of BI 2536 and γ-radiation when compared with controls and to individual treatment with either BI 2536 or irradiation, showing a true synergistic effect. In accordance with our results, Rodel et al.9 also demonstrated reduced clonogenicity in rectal tumor cells using PLK1 inhibition by siRNA combined with radiation. Decreased clonogenicity after treatment with BI 2536 alone has previously been reported by Renner et al.34 and by Morales et al.25 in leukemia and osteosarcoma cell lines, respectively, using different concentrations of BI 2536. In order to be able to metastasize and to have malignant progression, cells need to have the ability to colonize new environments. The clonogenic assay is a central test that shows the long-term survival and self-renewal of individual cells after treatment.36
When comparing the results of proliferation with clonogenic capacity, differences can be seen; however, this could be explained by the different methodologies. Once proliferation with XTT is measured, the metabolism of cells and clonogenic capacity only measure the number of colonies with more than 50 cells, reflecting the cell renewal of cells. Along with the fact that 2 Gy is not considered a high dose of radiation, it is possible to see a proliferation rate increase after this treatment.
GBM is a heterogeneous tumor, with variable responses to treatment. Here, we used seven GBM cell lines, and found that each one responded differently to BI 2536 and γ-radiation treatment; this could be mainly due to the difference in the PLK1 expression and cell metabolism (i.e., U251 expresses PLK1 gene two times more than U138 MG). GBM radioresistance has been suggested to be caused mainly by glioma stem-like cells; however, it is well known that the SF188 cell line has a high percentage (7%)37 and here, we found efficient inhibition of cell renewal (shown by colony formation) after treatment with 6 Gy, indirectly showing the effect of this drug on stem-like cells. In view of this, multimodality therapy for GBM has previously been demonstrated to improve a patient's survival, as has already been proposed by several authors and including the Canadian committee.36,38 Several phase II trials have shown synergistic or additive effects of many drugs with different mechanisms of action when combined with radiation and Temozolomide in GBM39–41 though, in practice, some of these tests have not been promising. Currently, new kinase inhibitors are being tested in combination with Temozolomide and radiation for cancer treatment; among them, it has been reported that Aurora kinase inhibitors could be suitable for use as chemo-adjuvant treatment in GBM.42 Most interestingly, different studies have shown a certain overlap between Aurora kinases and PLK1 coregulating several processes in mitosis, and the inhibition of either of them has been shown to cause similar phenotypes.43
In conclusion, PLK1 inhibition causes a decrease in proliferation and clonogenic formation, and an increase in apoptosis and sensitization to radiation. These results indicate the importance of PLK1 in GBM carcinogenesis, implying the possibility of using this kinase as a potential target to radiosensitize high-grade gliomas and to improve the actual treatment.
Acknowledgments
The authors are grateful to Leonardo L. Amaral for his cooperation in the irradiation of cell cultures and to Elza Tiemi Sakamoto-Hojo and Ana Paula de Lima Montaldi for facilitating cell cycle and apoptosis analysis. This research was supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico–Brazil) under grant number 471952/2011-7, and by FAPESP (Fundação de Apoio a Pesquisa do Estado de São Paulo) under grant number 2009/11053-2.
Discolsure Statement
The authors declare no conflict of interest.
References
- 1.Guckenberger M. Mayer M. Buttmann M, et al. Prolonged survival when temozolomide is added to accelerated radiotherapy for glioblastoma multiforme. Strahlenther Onkol. 2011;187:548. doi: 10.1007/s00066-011-2242-6. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert MR. Recurrent glioblastoma: A fresh look at current therapies and emerging novel approaches. Semin Oncol. 2011;38(Suppl 4):S21. doi: 10.1053/j.seminoncol.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Borchiellini D. Etienne-Grimaldi MC. Thariat J, et al. The impact of pharmacogenetics on radiation therapy outcome in cancer patients. A focus on DNA damage response genes. Cancer Treat Rev. 2012;38:737. doi: 10.1016/j.ctrv.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Argyriou AA. Antonacopoulou A. Iconomou G, et al. Treatment options for malignant gliomas, emphasizing towards new molecularly targeted therapies. Crit Rev Oncol Hematol. 2009;69:199. doi: 10.1016/j.critrevonc.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Strebhardt K. Multifaceted polo-like kinases: Drug targets and antitargets for cancer therapy. Nat Rev Drug Discov. 2010;9:643. doi: 10.1038/nrd3184. [DOI] [PubMed] [Google Scholar]
- 6.Schmit TL. Ledesma MC. Ahmad N. Modulating polo-like kinase 1 as a means for cancer chemoprevention. Pharm Res. 2010;27:989. doi: 10.1007/s11095-010-0051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winkles JA. Alberts GF. Differential regulation of polo-like kinase 1, 2, 3, and 4 gene expression in mammalian cells and tissues. Oncogene. 2005;24:260. doi: 10.1038/sj.onc.1208219. [DOI] [PubMed] [Google Scholar]
- 8.Chopra P. Sethi G. Dastidar SG, et al. Polo-like kinase inhibitors: An emerging opportunity for cancer therapeutics. Expert Opin Investig Drugs. 2010;19:27. doi: 10.1517/13543780903483191. [DOI] [PubMed] [Google Scholar]
- 9.Rodel F. Keppner S. Capalbo G, et al. Polo-like kinase 1 as predictive marker and therapeutic target for radiotherapy in rectal cancer. Am J Pathol. 2010;177:918. doi: 10.2353/ajpath.2010.100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerster K. Shi W. Ng B, et al. Targeting polo-like kinase 1 enhances radiation efficacy for head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2010;77:253. doi: 10.1016/j.ijrobp.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 11.Johnson EF. Stewart KD. Woods KW, et al. Pharmacological and functional comparison of the polo-like kinase family: Insight into inhibitor and substrate specificity. Biochemistry. 2007;46:9551. doi: 10.1021/bi7008745. [DOI] [PubMed] [Google Scholar]
- 12.Steegmaier M. Hoffmann M. Baum A, et al. BI 2536, a potent and selective inhibitor of polo-like kinase 1, inhibits tumor growth in vivo. Curr Biol. 2007;17:316. doi: 10.1016/j.cub.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 13.Tao Y. Zhang P. Frascogna V, et al. Enhancement of radiation response by inhibition of Aurora-A kinase using siRNA or a selective Aurora kinase inhibitor PHA680632 in p53-deficient cancer cells. Br J Cancer. 2007;97:1664. doi: 10.1038/sj.bjc.6604083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franken NA. Rodermond HM. Stap J, et al. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 15.Schmit TL. Zhong W. Setaluri V, et al. Targeted depletion of Polo-like kinase (Plk) 1 through lentiviral shRNA or a small-molecule inhibitor causes mitotic catastrophe and induction of apoptosis in human melanoma cells. J Invest Dermatol. 2009;129:2843. doi: 10.1038/jid.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruinsma W. Raaijmakers JA. Medema RH. Switching Polo-like kinase-1 on and off in time and space. Trends Biochem Sci. 2012;37:534. doi: 10.1016/j.tibs.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Dietzmann K. Kirches E. von B, et al. Increased human polo-like kinase-1 expression in gliomas. J Neurooncol. 2001;53:1. doi: 10.1023/a:1011808200978. [DOI] [PubMed] [Google Scholar]
- 18.Guan R. Tapang P. Leverson JD, et al. Small interfering RNA-mediated Polo-like kinase 1 depletion preferentially reduces the survival of p53-defective, oncogenic transformed cells and inhibits tumor growth in animals. Cancer Res. 2005;65:2698. doi: 10.1158/0008-5472.CAN-04-2131. [DOI] [PubMed] [Google Scholar]
- 19.Ellis PM. Chu QS. Leighl N, et al. A phase I open-label dose-escalation study of intravenous BI 2536 together with pemetrexed in previously treated patients with non–small–cell lung cancer. Clin Lung Cancer. 2013;14:19. doi: 10.1016/j.cllc.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Frost A. Mross K. Steinbild S, et al. Phase I study of the PLK1 inhibitor BI 2536 administered intravenously on three consecutive days in advanced solid tumours. Curr Oncol. 2012;19:e28. doi: 10.3747/co.19.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schoffski P. Polo-like kinase (PLK) inhibitors in preclinical and early clinical development in oncology. Oncologist. 2009;14:559. doi: 10.1634/theoncologist.2009-0010. [DOI] [PubMed] [Google Scholar]
- 22.Liu L. Zhang M. Zou P. Polo-like kinase 1 is essential to DNA damage recovery. Leuk Lymphoma. 2011;51:1079. doi: 10.3109/10428191003706939. [DOI] [PubMed] [Google Scholar]
- 23.Lu B. Mahmud H. Maass AH, et al. The PLK1 inhibitor BI 2536 temporarily arrests primary cardiac fibroblasts in mitosis and generates aneuploidy in vitro. PLoS One. 2010;5:e12963. doi: 10.1371/journal.pone.0012963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pezuk JA. Brassesco MS. Oliveira JC, et al. Antiproliferative in vitro effects of BI 2536-mediated PLK1 inhibition on cervical adenocarcinoma cells. Clin Exp Med. 2013;13:75. doi: 10.1007/s10238-011-0166-1. [DOI] [PubMed] [Google Scholar]
- 25.Morales AG. Brassesco MS. Pezuk JA, et al. BI 2536-mediated PLK1 inhibition suppresses HOS and MG-63 osteosarcoma cell line growth and clonogenicity. Anticancer Drugs. 2011;22:995. doi: 10.1097/CAD.0b013e32834a16d4. [DOI] [PubMed] [Google Scholar]
- 26.Zhang C. Sun X. Ren Y, et al. Validation of Polo-like kinase 1 as a therapeutic target in pancreatic cancer cells. Cancer Biol Ther. 2012;13:1214. doi: 10.4161/cbt.21412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mok WC. Wasser S. Tan T, et al. Polo-like kinase 1, a new therapeutic target in hepatocellular carcinoma. World J Gastroenterol. 2012;18:3527. doi: 10.3748/wjg.v18.i27.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagenblast J. Hirth D. Thron L, et al. Effects of the Polo-like-kinase-1-inhibitor BI2536 in squamous cell carcinoma cell lines of the head and neck. Oncol Lett. 2012;4:175. doi: 10.3892/ol.2012.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deckbar D. Jeggo PA. Lobrich M. Understanding the limitations of radiation-induced cell cycle checkpoints. Crit Rev Biochem Mol Biol. 2011;46:271. doi: 10.3109/10409238.2011.575764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He J. Qiu W. Zhang Z, et al. Effects of irradiation on growth and differentiation-related gene expression in osteoblasts. J Craniofac Surg. 2011;22:1635. doi: 10.1097/SCS.0b013e31822e5f66. [DOI] [PubMed] [Google Scholar]
- 31.Zhao Y. Jiang W. Li B, et al. Artesunate enhances radiosensitivity of human non-small cell lung cancer A549 cells via increasing NO production to induce cell cycle arrest at G2/M phase. Int Immunopharmacol. 2011;11:2039. doi: 10.1016/j.intimp.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 32.Bordon E. Leon LG. Rios-Luci C, et al. In vitro synergistic interaction between DTA0100 and radiation in human cancer cell lines. Anticancer Agents Med Chem. 2012;12:988. doi: 10.2174/187152012802650057. [DOI] [PubMed] [Google Scholar]
- 33.Heravi M. Tomic N. Liang L, et al. Sorafenib in combination with ionizing radiation has a greater anti-tumour activity in a breast cancer model. Anticancer Drugs. 2012;23:525. doi: 10.1097/CAD.0b013e32834ea5b3. [DOI] [PubMed] [Google Scholar]
- 34.Renner AG. Dos Santos C. Recher C, et al. Polo-like kinase 1 is overexpressed in acute myeloid leukemia and its inhibition preferentially targets the proliferation of leukemic cells. Blood. 2009;114:659. doi: 10.1182/blood-2008-12-195867. [DOI] [PubMed] [Google Scholar]
- 35.Nappi TC. Salerno P. Zitzelsberger H, et al. Identification of Polo-like kinase 1 as a potential therapeutic target in anaplastic thyroid carcinoma. Cancer Res. 2009;69:1916. doi: 10.1158/0008-5472.CAN-08-1693. [DOI] [PubMed] [Google Scholar]
- 36.Easaw JC. Mason WP. Perry J, et al. Canadian recommendations for the treatment of recurrent or progressive glioblastoma multiforme. Curr Oncol. 2011;18:e126. doi: 10.3747/co.v18i3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bax DA. Little SE. Gaspar N, et al. Molecular and phenotypic characterisation of paediatric glioma cell lines as models for preclinical drug development. PLoS One. 2009;4:e5209. doi: 10.1371/journal.pone.0005209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Butowski N. Chang SM. Lamborn KR, et al. Phase II and pharmacogenomics study of enzastaurin plus temozolomide during and following radiation therapy in patients with newly diagnosed glioblastoma multiforme and gliosarcoma. Neuro Oncol. 2011;13:1331. doi: 10.1093/neuonc/nor130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang SM. Lamborn KR. Malec M, et al. Phase II study of temozolomide and thalidomide with radiation therapy for newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2004;60:353. doi: 10.1016/j.ijrobp.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 40.Butowski N. Prados MD. Lamborn KR, et al. A phase II study of concurrent temozolomide and cis-retinoic acid with radiation for adult patients with newly diagnosed supratentorial glioblastoma. Int J Radiat Oncol Biol Phys. 2005;61:1454. doi: 10.1016/j.ijrobp.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 41.Prado RP. Pinfildi CE. Liebano RE, et al. Effect of application site of low-level laser therapy in random cutaneous flap viability in rats. Photomed Laser Surg. 2009;27:411. doi: 10.1089/pho.2008.2320. [DOI] [PubMed] [Google Scholar]
- 42.Borges KS. Castro-Gamero AM. Moreno DA, et al. Inhibition of Aurora kinases enhances chemosensitivity to temozolomide and causes radiosensitization in glioblastoma cells. J Cancer Res Clin Oncol. 2011;138:405. doi: 10.1007/s00432-011-1111-0. [DOI] [PubMed] [Google Scholar]
- 43.Macurek L. Lindqvist A. Medema RH. Aurora-A and hBora join the game of Polo. Cancer Res. 2009;69:4555. doi: 10.1158/0008-5472.CAN-09-0142. [DOI] [PubMed] [Google Scholar]