Abstract
Stromatal fruiting bodies of Cordyceps cardinalis were successfully produced in cereals. Brown rice, German millet and standard millet produced the longest-length of stromata, followed by Chinese pearl barley, Indian millet, black rice and standard barley. Oatmeal produced the shortest-length of fruiting bodies. Supplementation of pupa and larva to the grains resulted in a slightly enhanced production of fruiting bodies; pupa showing better production than larva. 50~60 g of brown rice and 10~20 g of pupa mixed with 50~60 mL of water in 1,000 mL polypropylene (PP) bottle was found to be optimum for fruiting body production. Liquid inoculation of 15~20 mL per PP bottle produced best fruiting bodies. The optimal temperature for the formation of fruiting bodies was 25℃, under conditions of continuous light. Few fruiting bodies were produced under the condition of complete darkness, and the fresh weight was considerable low, compared to that of light condition.
Keywords: Cereal, Cordyceps cardinalis, Environmental conditions, Fruiting body formation, Liquid inoculation, Silkworm pupa
Different Cordyceps species have been studied for in vitro production of fruiting bodies [1, 6]. Artificial fruiting body formation of Cordyceps and other entomopathogenic fungi has been tried using different organic substances [7, 9], and Kobayasi [1] was first to show that Cordyceps species can grow their fruiting bodies in rice. Since then, rice has been a regularly used growth medium of fruiting bodies of different Cordyceps species [3, 5, 10]. In addition to vegetal substances, alternative insects have been successfully inoculated and incubated under favorable conditions to produce fruiting bodies of Cordyceps and allied species [11, 14]. Besides the medium, optimum environmental conditions have been studied to grow Cordyceps species successfully [15, 17]. C. cardinalis was first reported from Japan and eastern United States [18]. It has been also recently reported from Korea. This study was carried out to find the optimum medium and environmental conditions for the growth of fruiting bodies of C. cardinalis. Cereals and silkworm pupa were used as the major components of the fruiting body formation medium, indicated in short as fruiting medium. Fruiting media were inoculated with liquid inoculum of the isolates and incubated for 60 days to observe the fruiting bodies. 50~60 g of brown rice, 10~20 g pupa and 50~60 mL of distilled water in 1,000 mL polypropylene (PP) bottle were found to be optimum for fruiting body production. 15~20 mL of liquid inoculum was ideal in the inoculation of fruiting medium and 25℃ was the most favorable temperature for fruiting body formation.
Materials and Methods
Fungal isolates and liquid inoculum preparation
Multi-ascospore isolates of C. cardinalis EFCC C-10376, preserved at the Entomopathogenic Fungal Culture Collection (EFCC), Kangwon National University, were used in this study. The specimen EFCC C-10376 was collected from Mt. Halla of Jeju-Do on July 12, 2003. Liquid inoculum was prepared in 100 mL of Sabouraud dextrose agar yeast (SDAY) broth (SDAY without agar) prepared in 250 mL Erlenmeyer flask, by inoculating five mycelial discs (5 mm) from three week old SDAY (dextrose 20 g, yeast extract 5 g, peptone 5 g and agar 15 g per 1,000 mL; pH 5.6) agar cultures of isolates, and were incubated for 7 days at 25 ± 1℃.
Fruiting medium
First, liquid inocula of the isolates were injected in eight different types of cereals to observe the formation of fruiting bodies relative to the grains. 50 g of cereal was mixed with 60 mL of distilled water in a 1,000 mL PP bottle and the mixture was sterilized at 121℃ for 20 min. 25 mL of liquid inoculum was inserted into each fruiting medium and was incubated at 20 ± 1℃ for 60 days under continuous light conditions and at 70~80% of relative humidity. Brown rice produced the highest amount of fruiting bodies and was thus used for the rest of the experiments. In order to select the optimum quantity of brown rice, fruiting bodies were produced in various amounts, ranging from 40~80 g per PP bottle. To observe the effect of silkworm, brown rice was supplemented by 10 g of pupa, 10 g of larva and a mixture of 5 g of each pupa and larva. Since pupa was found to yield better results than larva, brown rice was supplemented with various amounts of pupa, from 5~20 g, to as certain the optimal amount of pupa. Also, various amounts of distilled water, from 40~80 mL, were used to make fruiting medium to select the optimum amount. In each experiment, length and fresh weight of fruiting bodies were recorded after 60 days of incubation.
Effects of amount and pH level of liquid inoculum, temperature and light on fruiting body formation
Fruiting media with 50 g of brown rice and 10 g of pupa were injected with various amounts of liquid inoculum, ranging from 5~30 mL. Similarly, SDAY broth medium was adjusted to different pH levels, from 4.0~9.0, to grow the liquid inoculum in order to observe the effect of pH on fruiting body formation. Fruiting media were incubated at various temperatures, from 15~35℃, to observe the effect of temperature on fruiting body formation. To see the effect of light, the fruiting media were incubated under both continuous light and dark conditions.
Results and Discussion
Fruiting medium
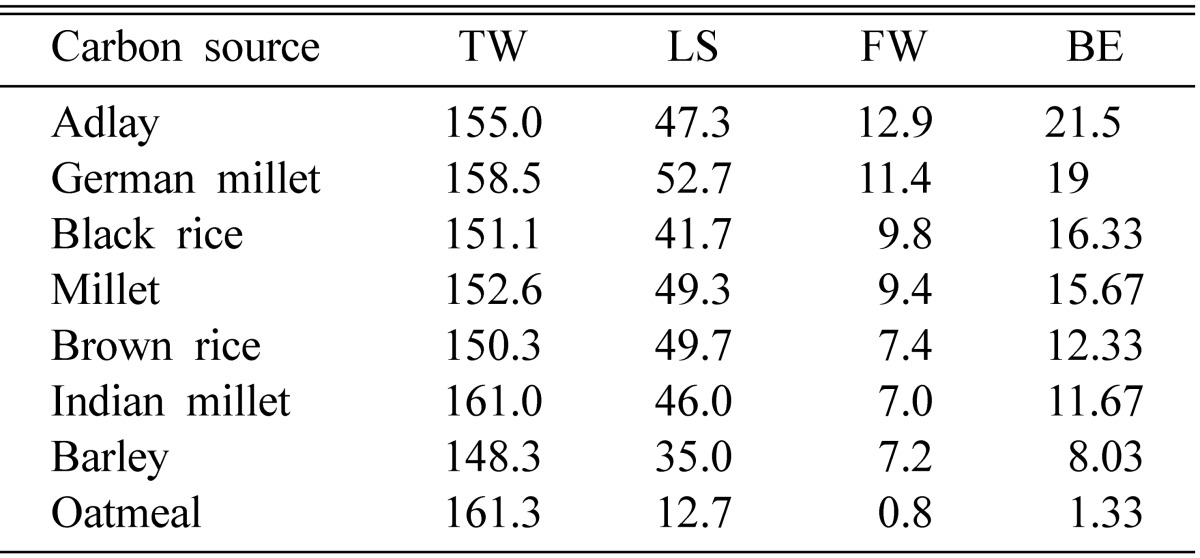
Of all the cereals used, brown rice produced the best fruiting bodies. Brown rice, German millet and millet showed higher stromata lengths, about 50 mm, followed by Chinese pearl barley (adlay), Indian millet, black rice and barley, which produced 35~47 mm-tall fruiting bodies (Table 1). Oatmeal produced the smallest fruiting bodies, of merely about 13 mm in length. Brown rice produced the highest amount of fresh weight of fruiting bodies, 6~7 g per PP bottle, followed by Chinese pearl barley, German millet and black rice. Oatmeal produced less than 1 g of fruiting bodies.
Table 1.
Effect of carbon source on fruit body formation of Cordyceps cardinalis
BE = FW/Medium weight × 100.
TW, total weight (g); LS, length of stroma (mm); FW, fruit body weight (g); BE, biological efficiency.
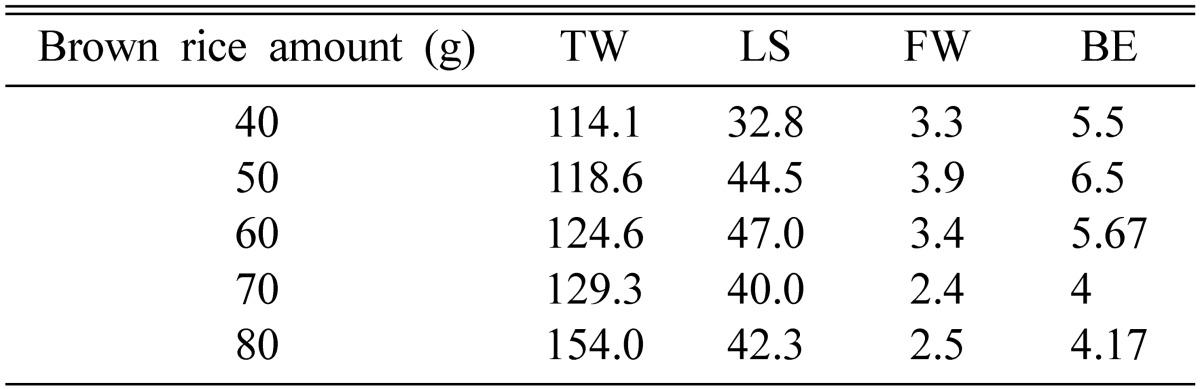
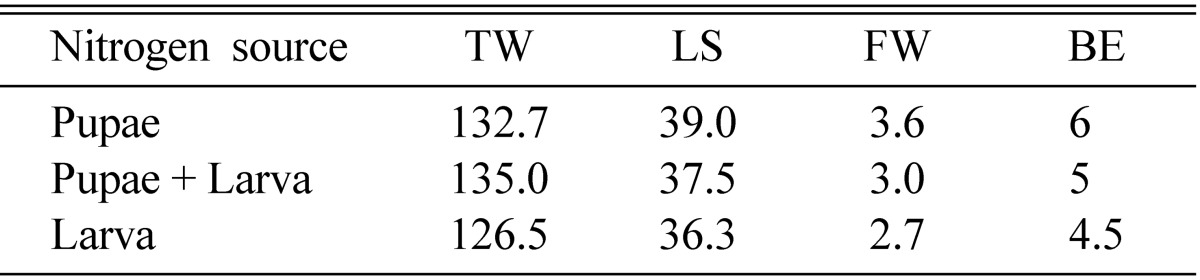
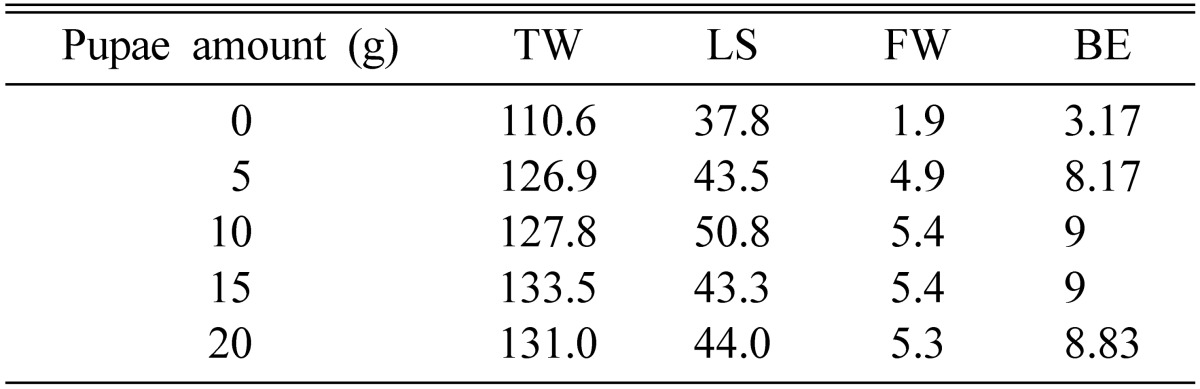
50~60 g of brown rice produced higher fruiting bodies as well as greater fresh weight (Table 3). It was interesting to note that 40 g of brown rice produced the shortest fruiting bodies, but the fresh weight of fruiting bodies was higher than those produced by 70~80 g of brown rice. Pupa produced higher fruiting bodies than did both larva and the mixture of pupa and larva (Table 2). 10 g of pupa produced fruiting bodies of about 40 mm in height, with a fresh weight of 3~4 g. 15~20 g of pupa produced shorter fruiting bodies but the fresh weights were similar to those produced by 10 g of pupa (Table 4). 5 g of pupa produced both shorter fruiting bodies and less fresh weight.
Table 3.
Effect of brown rice amount on fruit body formation of Cordyceps cardinalis
BE = FW/Medium weight × 100.
TW, total weight (g); LS, length of stroma (mm); FW, fruit body weight (g); BE, biological efficiency.
Table 2.
Effect of nitrogen source on fruit body formation of Cordyceps cardinalis
BE = FW/Medium weight × 100.
TW, total weight (g); LS, length of stroma (mm); FW, fruit body weight (g); BE, biological efficiency.
Table 4.
Effect of pupae amount on fruit body formation of Cordyceps cardinalis
BE = FW/Medium weight × 100.
TW, total weight (g); LS, length of stroma (mm); FW, fruit body weight (g); BE, Biological efficiency.
Fruiting bodies were most efficiently produced when 50 mL of distilled water was added to fruiting medium, resulting in stromata lengths of 5.5~6.0 mm and fruiting bodies weighing 5~6 g (Table 5). Higher amount of distilled water produced longer as well as more fresh weight of fruiting bodies, compared to 40 mL. The amount of water changes the hardness of the medium. At low water amount, mycelia cannot grow profusely during the course of incubation due to the hardness of the medium. Conversely, using a higher water amount the medium changes its texture and mycelia cannot grow deep into the medium, due to the low inter-grain space available for mycelial penetration. As a result, mycelia cannot utilize as efficiently the total nutrition available.
Table 5.
Effect of water amount on fruit body formation of Cordyceps cardinalis
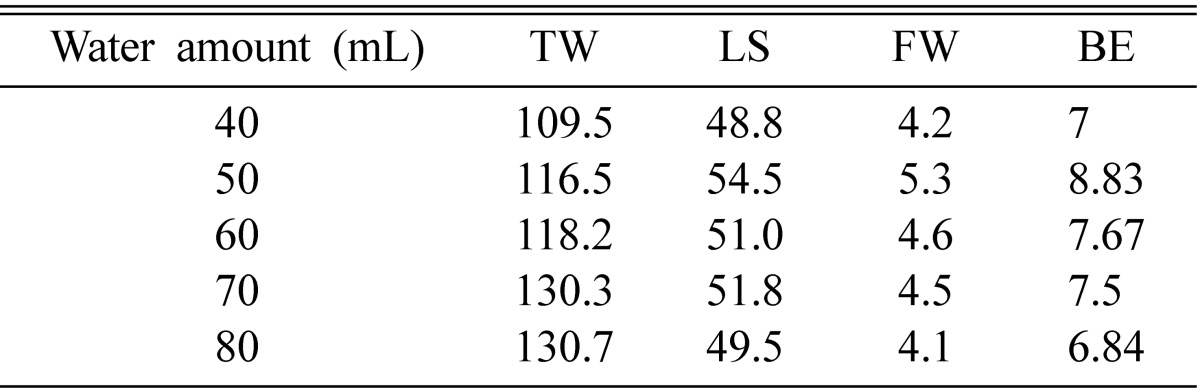
BE = FW/Medium weight × 100.
TW, total weight (g); LS, length of stroma (mm); FW, fruit body weight (g); BE, biological efficiency.
Effect of amount of inoculum and pH of broth medium on fruiting body formation
Fresh weight of the stromata was highest when injected with 15~20 mL of liquid inoculum (Table 6). When less inoculums was used it took a long time to grow over the medium, and sometimes could not cover the whole medium, consequently delaying the production of fruiting bodies. When a large quantity of inoculums was used, the mycelium grew more slowly, due to the presense of liquid in the medium. The faster mycelium grows on the medium, the higher the rate of stromata growth.
Table 6.
Effect of inoculum amount on fruit body formation of Cordyceps cardinalis
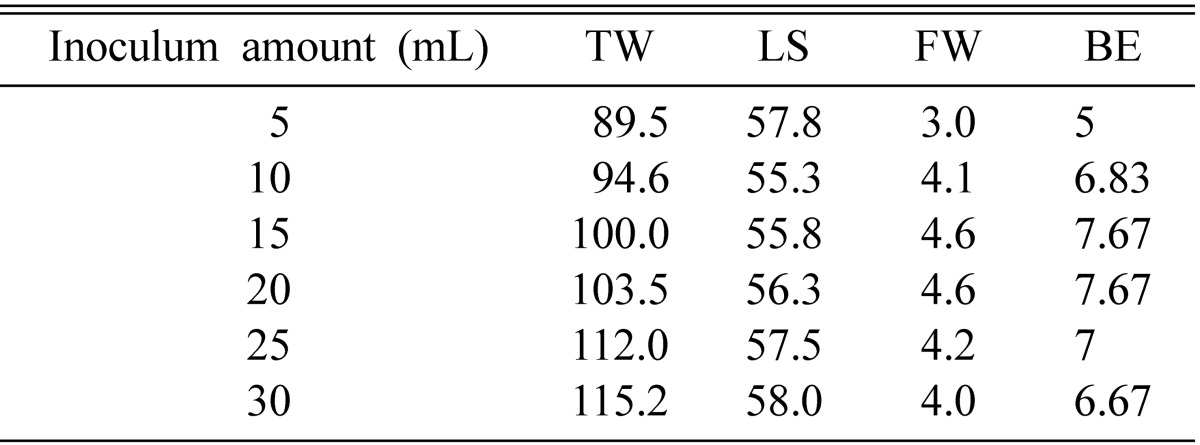
BE = FW/Medium weight × 100.
TW, total weight (g); LS, length of stroma (mm); FW, fruit body weight (g); BE, biological efficiency.
The initial pH level of 6.0 of liquid inoculum showed the longest stromata length and the highest fresh weight of fruiting bodies, followed by ranges from 5.0 to 9.0 (Table 7). The initial pH of 4.0 of the liquid inoculum produced the least fruiting bodies. It was previously shown that mycelial growth of C. cardinalis was highest when the pH of liquid medium was 7.0 and lowest at 4.0 [19]. Hence, the lowest fresh weight at pH of 4.0 might be due to less mycelial growth of the liquid inoculum. However, it has been shown that the pH of the liquid medium changed to around 5 after culture.
Table 7.
Effect of pH of liquid spawn on fruit body formation of Cordyceps cardinalis
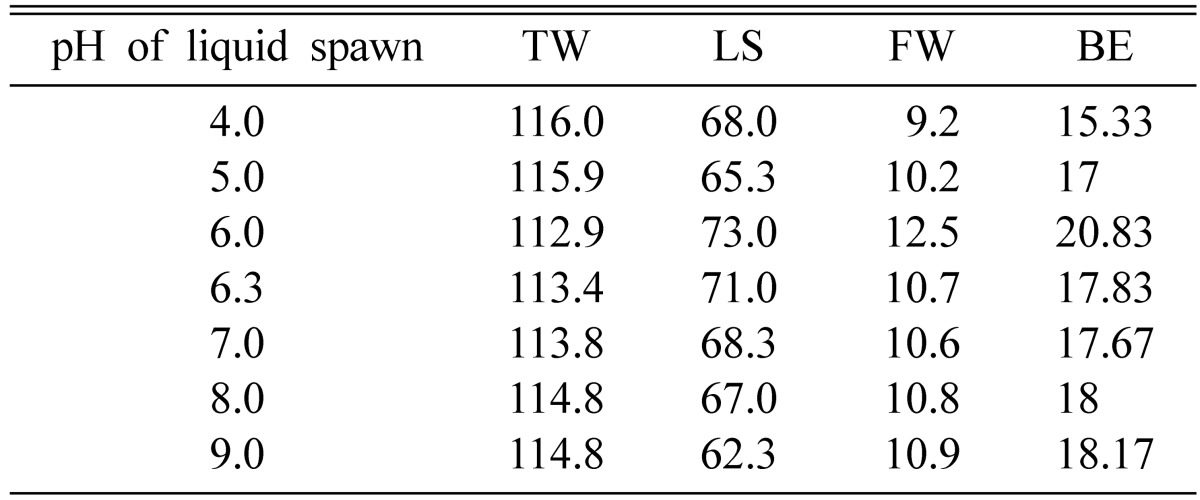
BE = FW/Medium weight × 100.
TW, total weight (g); LS, length of stroma (mm); FW, fruit body weight (g); BE, biological efficiency.
Effect of temperature and light on fruiting body formation
Highest fruiting bodies were formed at 25℃ (Table 8). Low temperatures of as low as 15℃ also produced fruiting bodies, however at high temperatures, 30℃ and above, no fruiting bodies were formed. Mycelia of C. cardinalis do not grow at higher temperatures. Our results in this respect followed, as no fruiting bodies could be formed at high temperatures. The fruiting medium also loses its water content during long incubation at high temperatures, and turns into hard biomass. Temperatures between 20~25℃ have been found to be optimal for fruiting body production [11, 12, 16, 19, 20].
Table 8.
Effect of temperature on fruit body formation of Cordyceps cardinalis
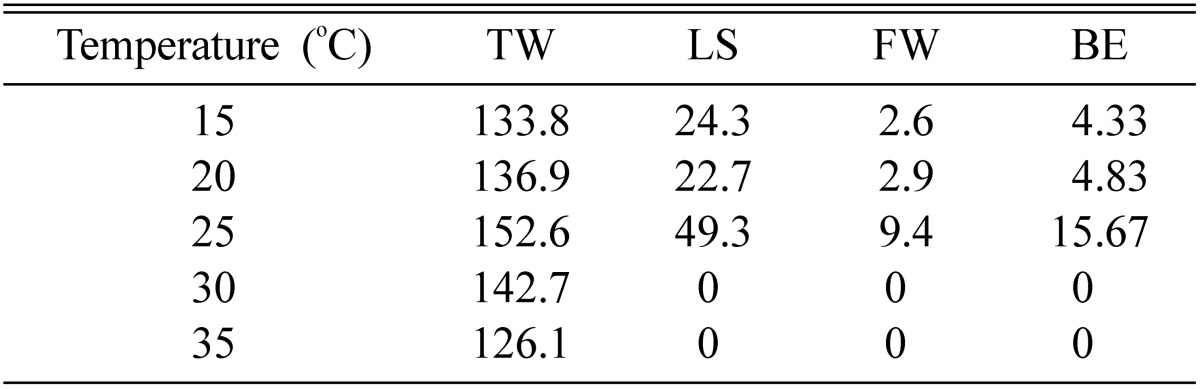
BE = FW/Medium weight × 100.
TW, total weight (g); LS, length of stroma (mm); FW, fruit body weight (g), BE, biological efficiency.
Under continuous white light conditions, fruiting bodies were formed vigorously whereas under complete dark conditions very few stromata were produced. The fresh weight of stromata was 9~10 g per PP bottle under light, but in dark conditions, less than 2 g of fruiting bodies were produced per PP bottle (Table 9). Light/dark periods have been found critical for fruiting body induction of Paecilomyces tenuipes [15], however continuous light condition without any dark period has successfully induced and sustained fruiting body production of Cordyceps [16, 17, 19, 20]. No fruiting bodies can be produced under continuous dark condition [12].
Table 9.
Effect of light on fruit body formation of Cordyceps cardinalis
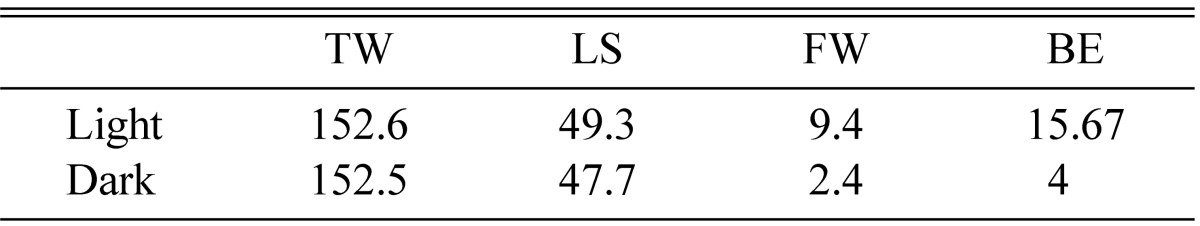
BE = FW/Medium weight × 100.
TW, total weight (g); LS, length of stroma (mm); FW, fruit body weight (g); BE, biological efficiency.
In conclusion, a mixture of 50~60 g of brown rice, 10~20 g of pupa and 50~60 mL of distilled water in 1,000 mL PP bottle were found to be the ideal conditions for fruiting body production of C. cardinalis. The amount of components of fruiting medium, however, is in fact relative to the shape and size of the fruiting bottle and thus can be adjusted accordingly. It was also observed that the fresh weight of fruiting bodies actually gave the total biomass of the fruiting bodies. Higher lengths with fewer fruiting bodies ultimately produced less biomass. A higher biomass of fruiting bodies is sometimes associated with abnormal shaped fruiting bodies, thereby decreasing their commercial value. Hence, more efforts are required to produce normal-shaped fruiting bodies with higher fresh weight.
Acknowledgements
The Authors wish to have acknowledgement of Cordyceps Research Institute, Kangwon National University for providing facilities to carry out this study.
References
- 1.Kobayasi Y. The genus Cordyceps and its allies. Sci Rept Tokyo Bunrika Daigaku Sect B. 1941;5:53–260. [Google Scholar]
- 2.Li CR, Nam SH, Geng DG, Fan MZ, Li ZZ. Artificial culture of seventeen Cordyceps spp. Mycosystema. 2006;25:639–645. [Google Scholar]
- 3.Liang Z. Anamorph of Cordyceps militaris and artificial culture of its fruitbody. Southwest China J Agric Sci. 1990;3:1–6. [Google Scholar]
- 4.Liu J, Liang Z, Liu A. Artificial culture of fruiting body of Cordyceps gunnii. Southwest China J Agric Sci. 1990;3:6–10. [Google Scholar]
- 5.Sung JM. The insects-born fungus of Korea in color. Seoul: Kyohak Publishing Co, Ltd.; 1996. [Google Scholar]
- 6.Wang SF, Zhu HX, Zhu P. The preliminary cultivation and observation on the vitality of Hirsutella sinensis. Edible Fungi China. 2003;22:4–6. [Google Scholar]
- 7.Basith M, Madelin MF. Studies on the production of perithecial stromata by Cordyceps militaris in artificial culture. Can J Bot. 1968;46:473–480. [Google Scholar]
- 8.Yahagi N, Kobayashi H, Yahagi R. Cultivation of Isaria japonica. Nippon Kingakkai News. 1993;20:6–8. [Google Scholar]
- 9.Yahagi N, Yahagi R, Takano F, Fushiya S, Tanaka T, Murakami K, et al. Growth of ascoscarps from cultured Cordyceps militaris (L.:Fr.) Fr. and Cordyceps formicarum Kobayasi in an agar medium. Nippon Kin Gakkai Kaiho. 2004;45:15–19. [Google Scholar]
- 10.Yamanaka K, Inatomi S, Hanaoka M. Cultivation characteristics of Isaria japonica. Mycoscience. 1998;39:43–48. [Google Scholar]
- 11.Harada Y, Akiyama N, Yamamoto K, Shirota Y. Production of Cordyceps militaris fruit body on artificially inoculated pupae of Mamestra brassicae in the laboratory. Trans Mycol Soc Jpn. 1995;36:67–72. [Google Scholar]
- 12.Sato H, Shimazu M. Stromata production for Cordyceps militaris (Clavicipitales: Clavicipitaceae) by injection of hyphal bodies to alternative host insects. Appl Entomol Zool. 2002;37:85–92. [Google Scholar]
- 13.Yahagi N. Cultivation of Isaria japonica and inoculation of silkworm. Touchu-Kasou. 1985;5:27–30. [Google Scholar]
- 14.Yu YX. Studies on artificial culture of Cordyceps sinensis. J Fungal Res. 2004;2:42–46. [Google Scholar]
- 15.Kanauchi A, Fukatsu T. Light-induced fruit body formation of an entomogenous fungus Paecilomyces tenuipes. Mycoscience. 1999;40:349–351. [Google Scholar]
- 16.Park GB, Park GB, Shrestha B, Sung JM. Optimal medium and cultural conditions for synnemata formation of Paecilomyces tenuipes. J Mush Sci Prod. 2004;2:60–68. [Google Scholar]
- 17.Sung JM, Choi YS, Shrestha B, Park YJ. Investigation on artificial fruiting of Cordyceps militaris. Korean J Mycol. 2002;30:6–10. [Google Scholar]
- 18.Sung GH, Spatafora JW. Cordyceps cardinalis sp. nov., a new species of Cordyceps with an east Asian-eastern North American distribution. Mycologia. 2004;96:658–666. [PubMed] [Google Scholar]
- 19.Lee JO, Shrestha B, Kim TW, Sung GH, Sung JM. Stable formation of fruiting body in Cordyceps bassiana. Mycobiology. 2007;35:230–234. doi: 10.4489/MYCO.2007.35.4.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shrestha B, Park YJ, Han SK, Choi SK, Sung JM. Instability in in vitro fruiting of Cordyceps militaris. J Mush Sci Prod. 2004;2:140–144. [Google Scholar]


