Table 1. The physicochemical properties of hydrophobic amino acids.
| glycine (gly) | alanine (ala) | valine (val) | leucine (leu) | isoleucine (ile) | |
|---|---|---|---|---|---|
| Molecular structure | 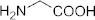 |
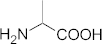 |
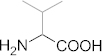 |
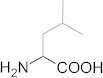 |
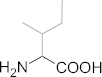 |
| Side chain | -H | -CH3 | -CH(CH3)2 | -CH2CH(CH3) | -CH(CH3)C2H5 |
| pKa1 (-COOH) at 273.15 K | 2.41 | 2.39 | 2.33 | 2.39 | 2.38 |
| Degree of ionization (-COOH) at pH 3.29 | 88.3% | 88.7% | 90.2% | 88.9% | 89.0% |
| pKa2 (-NH2) at 273.15 K | 10.32 | 10.43 | 10.34 | 10.33 | 10.41 |
| Degree of ionization (-NH2) at pH 3.29 | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% |
| Hydrophobicity | −0.4 | 1.8 | 4.2 | 3.8 | 4.5 |
