Figure 1. Segregation of APP and BACE-1 into distinct neuronal microdomains.
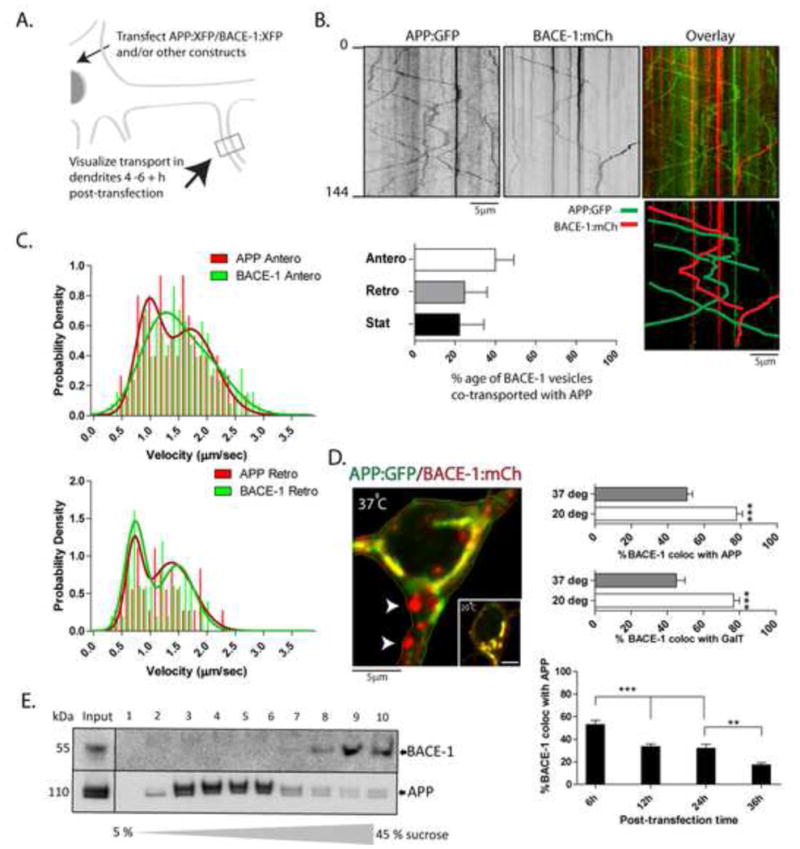
(A) Generic plan for transport experiments. Cultured hippocampal neurons were transfected with tagged APP/BACE-1 (or other) constructs, vesicle-transport in dendrites was imaged live after 4–6 h, and resultant kymographs were analyzed (see methods for more details).
(B) Representative kymographs from simultaneous APP:GFP and BACE-1:mCherry imaging. Pseudocolor overlay of the two kymographs (top right), with mobile tracks marked (below right). Note little overlapping of mobile and stationary APP and BACE-1 vesicles, quantified in the bar-graph below.
(C) Velocity-histograms of mobile APP and BACE-1 vesicles were best-fitted by two ‘peaks’ (arrowheads – also see Tang et al., 2012), with subtle differences in anterograde transport kinetics. The distribution of 1st and 2nd anterograde peaks for APP and BACE was significantly different (APP: 0.33/0.67; BACE: 0.5/0.5; 1st and 2nd peak respectively; p = .0216), while distribution of 1st and 2nd retrograde peaks was not (APP: 0.29/0.71; BACE: 0.45/0.55, 1st and 2nd peak respectively; p = 0.2549).
(D) Neurons were transfected with BACE-1:mCherry and APP:GFP as above, and BACE-1/APP distribution in the soma was analyzed after 4–6h. Note that although a large fraction of the BACE-1 co-localizes with APP in a perinuclear ER→Golgi pattern, vesicles containing BACE-1 (but no APP) are also seen (arrowheads). Incubation of neurons at 20°C for 2h (expected to block exit of vesicles from the Golgi) greatly increases BACE-1/APP co-localization. Similar results were obtained with BACE-1 and GalT – a Golgi-marker. Over time, the extent of APP/BACE-1 colocalization was also diminished, further suggesting differential redistribution of these two proteins after biogenesis. n ≈ 15 neurons analysed for each temperature, from at least two separate cultures. *p < 0.05; **p < 0.01; ***p < 0.001; two tailed, unpaired t-test. (E) Sucrose density gradients from ‘vesicle-pellet’ fractions of mouse brains (see text) show that endogenous APP and BACE-1 is also largely segregated in-vivo.
