Table 1.
Inhibition constants for the interaction of immucillins with E. coli MTAN and human MTAPa and the ratio of the two values in order to provide a qualitative indication of the therapeutic window for the treatment of an E. coli infection.
| Compound |
Ki for E. coli MTAN (nM) |
Ki for Human MTAP (nM) |
Ki Human MTAP / Ki E. coli MTAN |
|---|---|---|---|
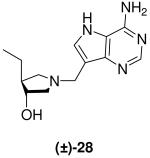
|
0.84 ± 0.06 | *3.2 ± 0.2 | 4 |
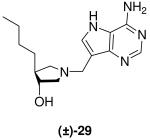
|
*0.009 ± 0.001 | 0.8 ± 0.1 | 89 |
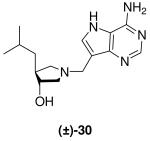
|
*0.047 ± 0.009 | *1.7 ± 0.3 | 36 |
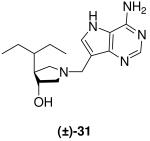
|
0.7 ±0.1 | 18 ± 2 | 26 |
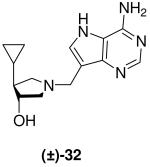
|
0.063 ± 0.005 | *2.1 ± 0.3 | 33 |
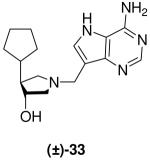
|
*0.013 ± 0.001 | *2.6 ± 0.4 | 200 |
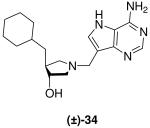
|
0.059 ± 0.008 | 14 ± 2 | 237 |
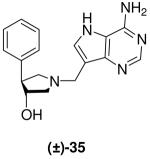
|
*0.03 ± 0.002 | 8 ± 1 | 267 |
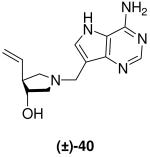
|
0.65 ± 0.03 | 3.2 ± 0.4 | 4.9 |
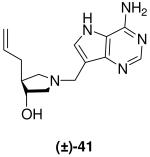
|
0.35 ± 0.03 | 3.0 ± 0.3 | 8.6 |
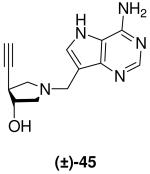
|
0.39 ± 0.02 | 31 ± 3 | 80 |
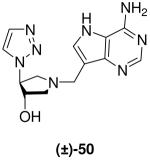
|
2.0 ± 0.2 | 59 ± 8 | 30 |
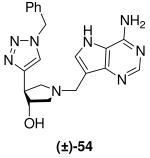
|
*0.064 + 0.005 | -b | selective |
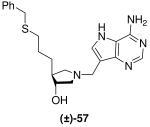
|
*0.054 ± 0.005 | 71 ± 5 | 1314 |
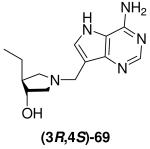
|
0.31 ± 0.02 | *0.7 ± 0.2 | 2 |
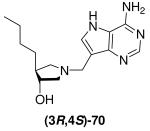
|
0.0034 ± 0.0009 | *0.55 ± 0.07 | 162 |
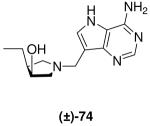
|
1.8 ± 0.3 | 34 ± 3 | 19 |
Ki is the dissociation constant for the first step in E + I ↔ EI ↔ EI*, the two-step binding characteristic of slow-onset tight-binding inhibition.
indicates slow onset binding and is theoverall dissociation constant for E + I ↔ EI*. In cases where no * is shown then slow-onset inhibition was not observed.
indicates that no inhibition was observed at 2.5 μM.
