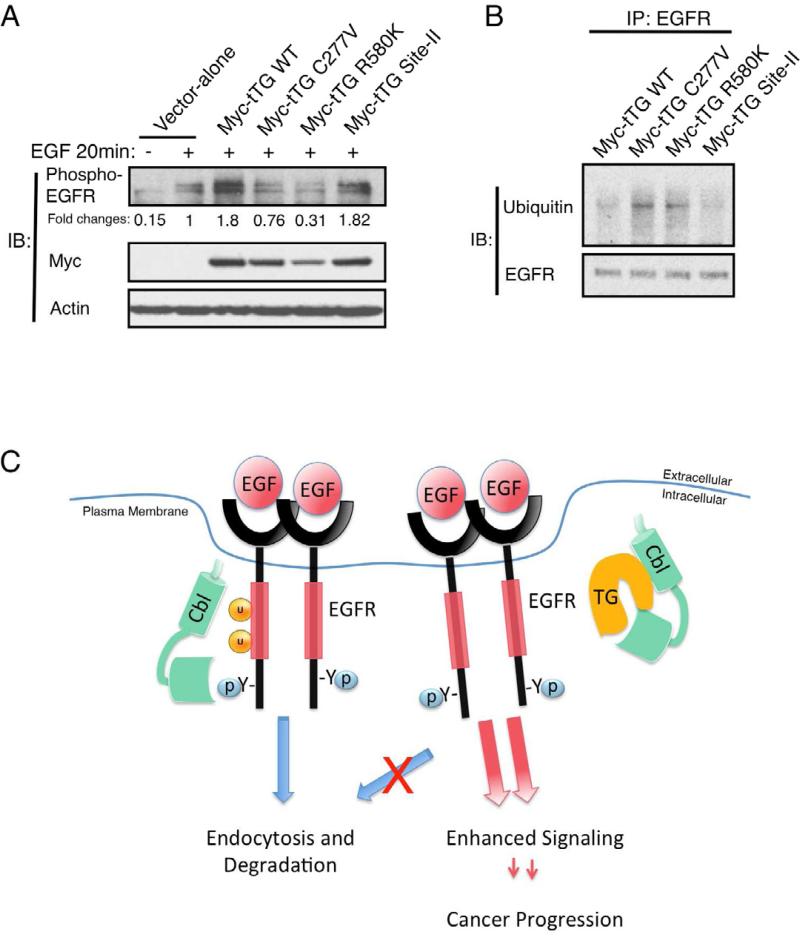
Figure 7. Ectopic expression of tTG forms that are in the “closed” conformation enhances EGFR signaling.
(A) Cell extracts from U87 cells ectopically expressing the vector-alone, wild-type tTG (Myc-tTG WT), or one of the different mutant forms of tTG stimulated without or with EGF (100 ng/ml) for 20 min, were immunoblotted (IB) using phospho-EGFR, Myc, and actin antibodies.
(B) Immunoprecipitations with an EGFR antibody (IP: EGFR) were performed on the cell extracts from SKBR3 cells transfected with wild-type (Myc-tTG WT) or one of the different mutant forms of tTG indicated. The resulting immuno-complexes were then immunoblotted (IB) with ubiquitin and EGFR antibodies.
(C) Schematic representation depicting the effects of tTG on EGFR signaling. In normal conditions, c-Cbl is recruited to the activated EGFR and mediates its ubiquitylation. The ubiquitylated EGFR is then endocytosed and targeted for degradation in the lysosome. In brain tumor cells where tTG is abundantly expressed, tTG forms a complex with c-Cbl and prevents it from ubiquitylating the EGFR. This disrupts the normal down-regulation of the EGFR, extending its signaling lifetime and enhancing cellular transformation.
See also Figure S5.