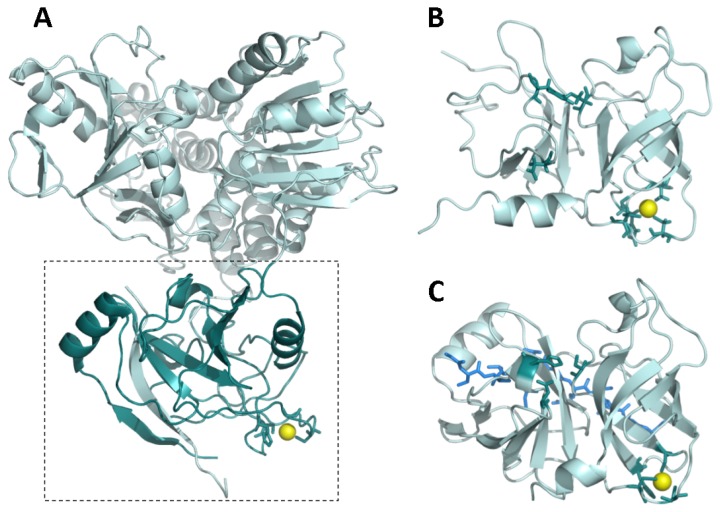
Figure 2.
(A) Full-length NS3 protein from hepatitis C virus (PDB code: 1CU1) [26]. The dashed rectangle delimitates the protease domain (dark cyan). The zinc ion is shown as a yellow sphere. The NS4A cofactor-mimicking peptide incorporates into the NS3 protease domain as an additional beta strand (cyan); (B) NS3 protease domain in the absence of NS4A cofactor (PDB code: 1BT7) [27]. The zinc ion is shown as a yellow sphere. The zinc-coordinating residues and the catalytic residues are shown as dark cyan sticks; (C) NS3 protease domain in the presence of a NS4A cofactor-mimicking peptide (blue sticks) (PDB code: 1JXP) [28]. The zinc ion is shown as a yellow sphere. The zinc-coordinating residues and the catalytic residues are shown as dark cyan sticks. The NS4A cofactor-mimicking peptide incorporates into the NS3 protease domain as an additional beta strand (blue sticks). Comparison between (B) and (C) reveals a structural rearrangement affecting the N-terminal domain of NS3 protease upon NS4A binding and propagating to the catalytic triad (for example, D81 is reoriented upward, towards a productive conformation).