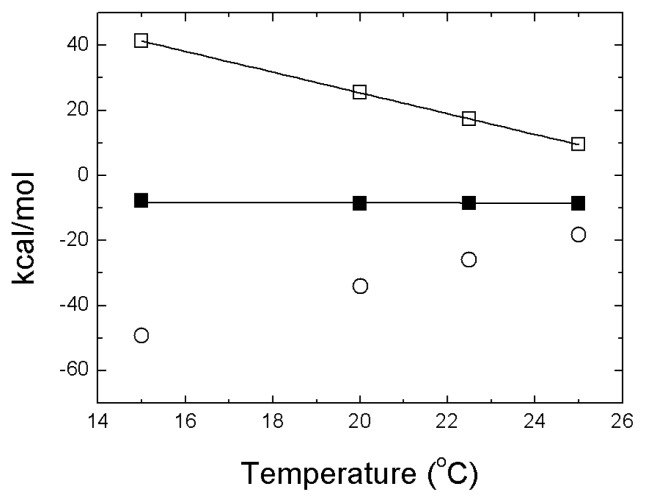
Figure 3.
Thermodynamic dissection of the NS3 protease-zinc interaction. Temperature dependence of the Gibbs energy (ΔG, closed squares), enthalpy (ΔH, open squares) and entropy (−TΔS, open circles) for zinc binding to NS3 protease determined by ITC at pH 5. The lines correspond to the global non-linear regression fits for the temperature dependency of the Gibbs energy and enthalpy of interaction, considering a constant binding heat capacity. The strong temperature dependencies of enthalpy and entropy of binding suggest a considerable structural rearrangement coupled to metal ion binding.