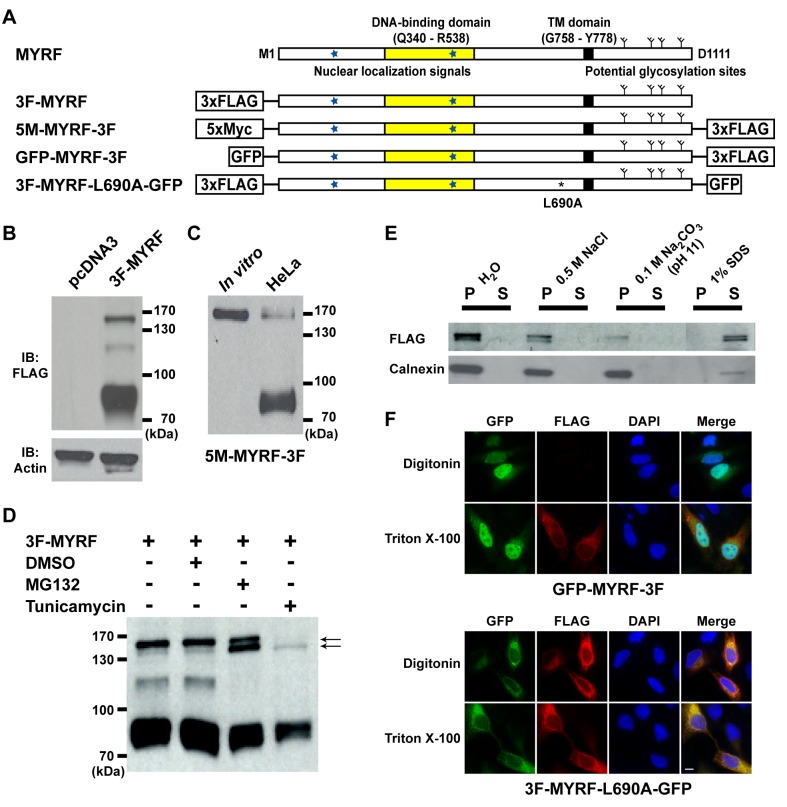
Figure 2. Full-length MYRF is a type-II membrane protein.
(A) Predicted sequence features of MYRF and sequence diagrams of various MYRF constructs used for experiments. (B) Western blot of HeLa cells transfected with pcDNA3 and 3F-MYRF. (C) The top band of HeLa cells that were transfected with 5M-MYRF-3F has the same electrophoretic mobility as full-length protein products for the same construct that were obtained with an in vitro translation system. (D) Full-length forms of MYRF consist of two closely spaced bands that represent glycosylated and unglycosylated full-length MYRF, respectively (indicated by the two arrows). (E) HeLa cells transfected with 3F-MYRF were disrupted using a Dounce-type homogenizer, and then centrifuged at 200× g for 5 min to obtain a supernatant fraction. It was mixed with 0.1 volume of each of the following chemicals: 5 M NaCl, 1 M Na2CO3 (pH 11), and 10% SDS. After incubation for 20 min at room temperature, mixtures were centrifuged at 20,000× g for 15 min at 4°C to separate supernatant (S) from pellet (P). Calnexin, a known integral membrane protein, served as a control. (F) Membrane topology of GFP-MYRF-3F and 3F-MYRF-L690A-GFP in HeLa cells. When cell membranes were selectively permeated by digitonin, FLAG IF signals of GFP-MYRF-3F could not be detected, indicating that the C-terminus of MYRF is located within the ER lumen. In contrast, FLAG IF signals of 3F-MYRF-L690A-GFP were robustly detected even when cell membranes were selectively permeated by digitonin, indicating that the N-terminus of full-length MYRF is located on the cytoplasmic side of ER membranes. Scale bar, 10 µm.