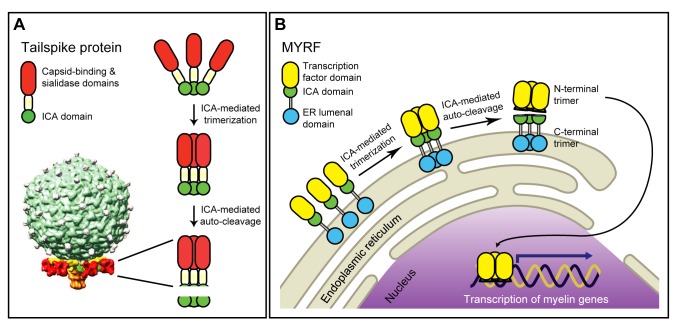
Figure 6. The ICA domain catalyzes trimerization-dependent auto-proteolysis in entirely distinct protein and cellular contexts.
(A) In K1F bacteriophage, the C-terminal ICA domain within each tailspike endosialidase auto-catalytically removes itself following tailspike trimerization, guiding maturation of the six tailspikes surrounding the phage tail (shown for phage K1E, adapted from [55]). (B) Once generated as a type-II membrane protein, the ICA domain is thought to induce the trimerization of MYRF, upon which it cleaves itself, generating two independent trimers. The N-terminal trimer translocates to the nucleus and activates the transcription of myelin genes by direct DNA binding. The transcriptional role of the N-terminal trimer serves to promote the terminal differentiation of OLs, likely aided by an as-yet-unknown function of the C-terminal trimer that remains in the ER.