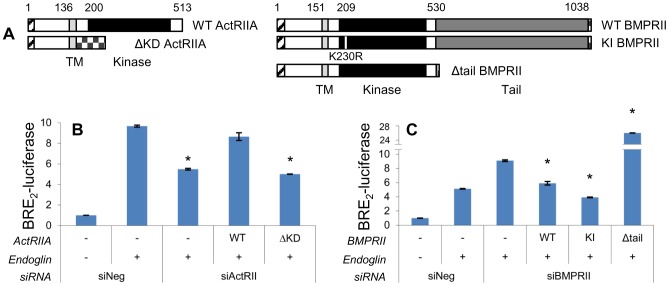
Figure 4. ActRIIA promotion of Smad1 signaling is kinase dependent, while BMPRII inhibition occurs via the tail domain.
A) Schematic depiction of ActRIIA and BMPRII constructs. Signal peptide (hatched), transmembrane domain (light gray), kinase domain (black), and BMPRII tail domain (dark gray) are indicated with the amino acid position that begins each portion. Also indicated are five sequential Myc tags or single FLAG tag at the C-terminus of ΔKD ActRIIA and BMPRII constructs, respectively (checkered). The small white stripe in KI BMPRII’s kinase domain represents the site of kinase-inactivating mutation. Segment lengths are to scale. B) ActRIIA promotes Smad1 signaling dependent on the kinase domain. PC3-M cells were transfected with BRE2-luciferase and Renilla luciferase, endoglin, wild type (WT) or kinase domain deletion (ΔKD) ActRIIA constructs, and siRNA to ActRIIA or non-targeting as indicated. Luciferase assay performed as in Figure 2B. Data represent mean ± SD of a single representative experiment (N = 2 replicates), repeated twice (N = 2 replicates) with similar results. *, p≤0.05 compared to Eng/siNeg. C) BMPRII suppresses Smad1 signaling independent of kinase function but dependent on the tail domain. PC3-M cells were transfected as above except that BMPRII constructs and siRNA were used. WT = wild type; KI = kinase inactive; Δtail = tail domain deleted. Luciferase assay performed as in Figure 2B. Data represent mean ± SD of a single representative experiment (N = 2 replicates), repeated twice (N = 2 replicates) with similar results. *, p≤0.05 compared to Eng/siBMPRII.