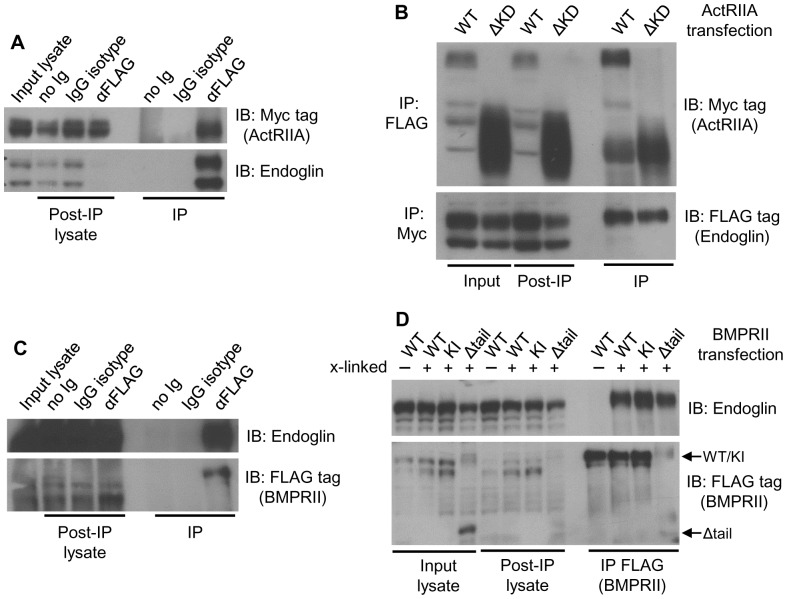
Figure 7. Endoglin physically interacts with ActRIIA and BMPRII.
After transient transfection, the surface proteins of PC3-M cells were crosslinked, cells lysed, immunoprecipitation performed, crosslinking reversed, and Western blot performed. A) ActRIIA coprecipitates with endoglin. Cells were transfected with Myc-ActRIIA and FLAG-endoglin, FLAG (endoglin) immunoprecipitated, and Western blots probed for ActRIIA (with anti-Myc) and endoglin. Controls for immunoprecipitation included agarose beads alone (no Ig) and nonspecific isotype control IgG (IgG isotype). Input lysate, lysate post-immunoprecipitation (i.e. supernatant), and immunoprecipitation (IP) samples were loaded as indicated. Data are from a representative experiment (N = 2 experiments). B) The kinase domain of ActRIIA is dispensable for interaction with endoglin. Cells transfected with Myc-WT or -ΔKD-ActRIIA and FLAG-endoglin as indicated, FLAG or Myc was immunoprecipitated as indicated, and Western blots probed as indicated. Data are from a representative experiment (N = 4 experiments). (C) BMPRII precipitates with endoglin. Cells were transfected with FLAG-BMPRII and untagged endoglin, FLAG immunoprecipitated, and Western blots probed as indicated. Data are from a representative experiment (N = 2 experiments). (D) The kinase activity and tail domain of BMPRII are dispensable for interaction with endoglin. Cells transfected with FLAG-WT, -KI, or -Δtail-BMPRII and untagged endoglin as indicated, FLAG-BMPRII immunoprecipitated, and Western blots probed as indicated. In some instances surface proteins were crosslinked (+), which was reversed after immunoprecipitation, while in other instances proteins were not crosslinked (-) Data are from a representative experiment (N = 3 experiments).