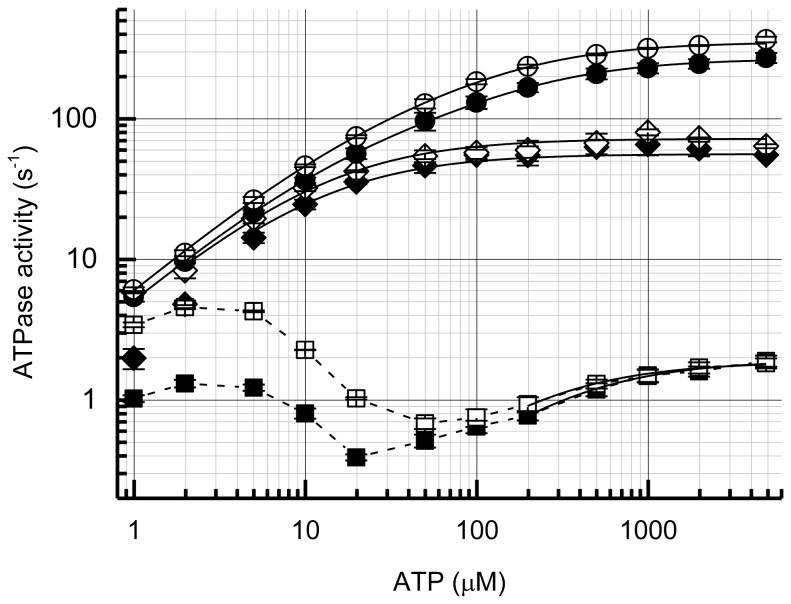
Figure 2. Dependence of BF1 ATPase activity on ATP concentration.
The ATPase activities of initial (closed diamonds; α3β3γ, and open diamonds; α3β3γε), steady-state (closed squares; α3β3γ, and open squares; α3β3γε) and in the presence of LDAO (closed circles; α3β3γ, and open circles; ATPase activities of α3β3γε) at each ATP concentration was calculated from the velocities at 2-7 s, 12–13 min after the start of the reaction, and 100–150 s after the addition of LDAO, respectively. Error bars represent standard errors. The solid lines were fitted to a single (initial and steady-state) or sum of two (in the presence of LDAO) Michaelis–Menten equation(s). Only data from 200 µM and the above concentrations of ATP were used to fit the steady-state rates of α3β3γ and α3β3γε. Data from 1 µM (and 2µM, in the case of α3β3γ) were not used to fit the initial rate. The K M and the associated k cat values are 12.7 ± 0.9 µM, 56.2 ± 0.9 s-1 (α3β3γ, initial); 13.8 ± 0.9 µM, 72.3 ± 1.3 s-1 (α3β3γε, initial); 296 ± 25 µM, 1.92 ± 0.06 s-1 (α3β3γ, steady-state); 209 ± 18 µM, 1.87 ± 0.04 s-1 (α3β3γε, steady-state); 16.0 ± 1.9 µM, 68.8 ± 10.9 s-1 and 184 ± 32 µM, 199 ± 10 s-1 (α3β3γ, +LDAO); and 18.7 ± 3.4 µM, 80.1 ± 19.2 s-1 and 138 ± 18 µM, 272 ± 18 s-1 (α3β3γε, +LDAO).