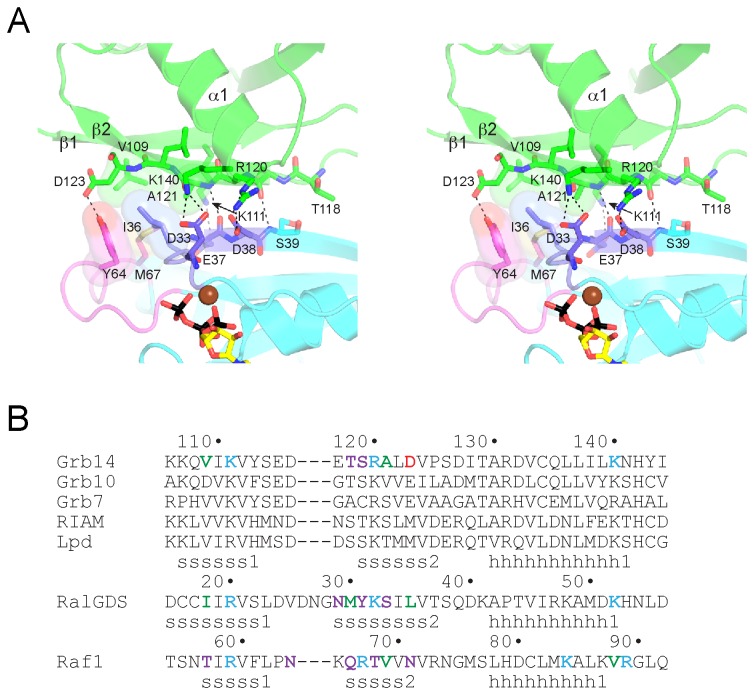
Figure 2. Mode of binding of Grb14RA-PH to H-Ras.
(A) Stereo diagram of the binding interface. The Grb14 RA domain is colored green, and H-Ras is colored cyan, with switch 1 colored purple and switch 2 colored magenta. Select side-chain and backbone atoms are shown in stick representation, with oxygen atoms colored red and nitrogen atoms colored blue. Mg-GTP is colored as in Figure 1. Select hydrogen bonds and salt bridges are represented by black dashed lines, and van der Waals interactions by side-chain surfaces. (B) Structure-based sequence alignment of RA domains. The top set of sequences (human) are for the RA-PH-containing proteins: Grb7-10-14, RIAM, and lamellipodin (Lpd). Residues that interact with Ras (or Rap1 in the case of Raf1) are colored red for acidic, cyan for basic, violet for non-charged polar, or green for hydrophobic. The secondary structure assignments (s, β strand; h, α helix) for the three structures are shown below the sequences.