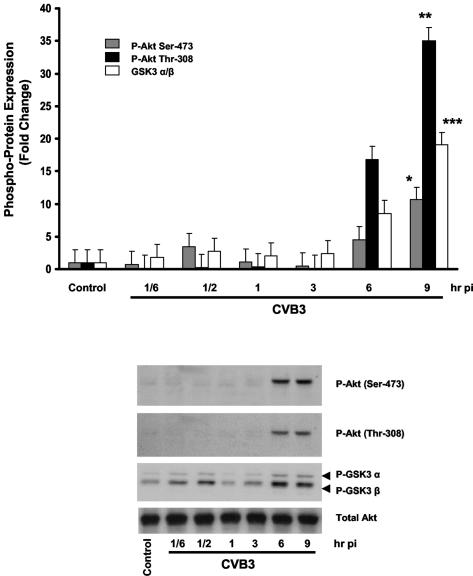
FIG. 1.
CVB3 infection leads to phosphorylation and activation of Akt on both serine 473 and threonine 308 residues. Following serum starvation for 24 h, HeLa cells were either sham or CVB3 infected (MOI of 10) for 1 h. Cell lysates were collected at the specified times postinfection (pi) and subjected to Western blotting to detect phosphorylation of Akt on both Ser-473 and Thr-308 sites. The activity of phospho-Akt was determined based on the phosphorylation of glycogen synthase kinase-3 α/β (GSK3 α/β), a downstream substrate of PKB. Total Akt protein was measured to ensure equal protein loading. Protein density is expressed as the increase in the level of phosphorylated protein with respect to the noninfected control (*, **, and ***, P < 0.01). The data shown are representative of those from quadruplicate experiments. Error bars indicate standard deviations.