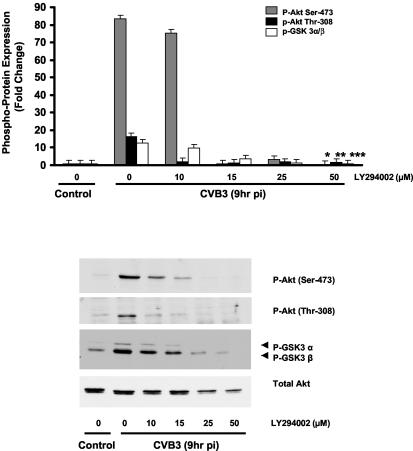
FIG. 2.
CVB3 induces Akt phosphorylation via a PI3K-dependent mechanism. HeLa cells were serum starved for 24 h. After being treated with LY294002 (50 μM) or vehicle (DMSO), cells were infected with either CVB3 (MOI of 10) or DMEM for 1 h. Total protein was extracted and analyzed by immunoblotting. Akt phosphorylation on both Ser-473 and Thr-308 residues was prevented by the PI3K inhibitor, indicating that Akt activation by CVB3 was mediated via a PI3K-dependent pathway. Total Akt protein was detected to ensure equal loading protein. Density values for phosphoproteins are expressed as the fold change compared to nontreated CVB3-infected cells (*, **, and ***, P < 0.01). GSK 3α/β, glycogen synthase kinase-3 α/β; pi, postinfection. Data are representative of those from two independent experiments. Error bars indicate standard deviations.