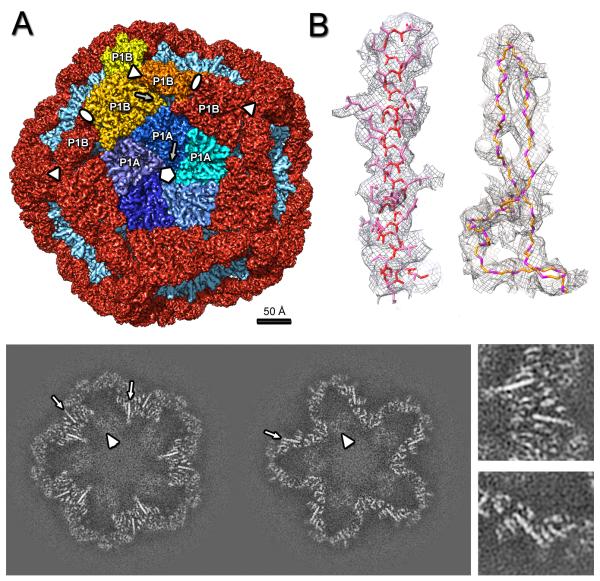
Figure 2.
Cryo-EM reconstruction of the ϕ6 procapsid. (A) Segmentation of the outer surface viewed along a 5-fold axis. The 12 inverted 5-fold vertices are occupied by P1A pentamers (the 5 subunits are in shades of blue) set in a dodecahedral frame of sixty P1B subunits (red, except for the three subunits around one 3-fold axis which are in shades of yellow). (B) Density for one α-helix (left) and two β-strands with the corresponding atomic model (side-chains are shown for the α-helix only, for clarity). (C) Slices through the cryoEM reconstruction viewed along the 5-fold axis at 20 Å (left) and 40 Å (right) from the procapsid center. Elongated densities representing α-helices that are approximately in-plane are indicated by arrows and enlarged in the right panels. Scale bar: 100 Å. See also Movie S2.