Abstract
Myelinating Schwann cells express distinct sensory and motor phenotypes as defined by their differing patterns of growth factor production (Hoke et al., 2006). The heterogeneous growth factor requirements of sensory and motor neurons, however, suggest that Schwann cell phenotype might vary across a broad spectrum. To explore this possibility, we selectively denervated six discrete Schwann cell populations: dorsal root, cutaneous nerve, cutaneous unmyelinated axons, muscle nerve afferents, muscle nerve efferents, and ventral root. Real-time RT-PCR for 11 growth factors was performed on the 6 target Schwann cell populations 5, 15, and 30 days after their denervation, and on normal cutaneous nerve, muscle nerve, ventral root, and dorsal root to establish baseline expression levels. Within the denervated axon populations, IGF-1 and VEGF were expressed most prominently in cutaneous nerve, HGF, NGF, and BDNF in cutaneous nerve and dorsal root, GDNF in dorsal root and ventral root, PTN in ventral root and muscle nerve efferents, and IGF-2 in both afferents and efferents within muscle nerve; expression of CNTF, FGF-2 and NT-3 was not modality or location specific. ELISA for NGF, BDNF, and GDNF confirmed that gene expression correlated with protein concentration. These findings demonstrate that growth factor expression by denervated Schwann cells is not only subject to further regulation within the previously-defined sensory and motor groups, but also varies along a central-peripheral axis. The traditional view of myelinating Schwann cells as a homogenous population is modified by the realization that complex regulation produces a wide variety of Schwann cell phenotypes. Additionally, we found that Schwann cell phenotype is maintained for 2 weeks in vitro, demonstrating that it may survive several cell divisions without instructive cues from either axons or basal lamina.
Keywords: denervation, peripheral nerve, nerve growth factor, brain derived neurotrophic factor, glial derived neurotrophic factor, pleitrophin, vascular endothelial growth factor, hepatocyte growth factor, insulin-like growth factor
Schwann cell phenotype is usually described as either myelinating or non-myelinating (Jessen and Mirsky, 2002). Recently, however, Schwann cells from cutaneous nerve and ventral root have been found to express distinct patterns of growth factors (Hoke et al., 2006), a finding confirmed recently by Jesuraj et al. (2012). We now present evidence that Schwann cell phenotype is even more varied, is regulated by both the modality of associated axons and by central/peripheral location, and persists through several Schwann cell divisions in vitro
Early studies of nerve regeneration suggested that sensory and motor pathways might have unique identities (Langley and Anderson, 1904; Ramon y Cajal, 1928). A clear functional difference between sensory and motor nerve was not unmasked, however, until experiments were designed to provide regenerating motor axons with equal access to both environments. Under these conditions, motor axons preferentially reinnervated muscle nerve, even when access to muscle was denied (Brushart, 1993). Motoneurons were also found to support more myelinated collaterals in cutaneous nerve than in muscle nerve, indicating a modality-specific difference in pathway stimulation and/or maintenance of collateral sprouting (Redett et al., 2005). Cutaneous and muscle nerve thus differ in ways that can be detected by regenerating motor axons and that can modify their subsequent behavior.
We recently examined growth factor expression by ventral root and cutaneous nerve in response to denervation and after reinnervation with cutaneous or motor axons (Hoke et al., 2006). The expression of growth factor genes differed strikingly between these prototypical sensory and motor nerves, resulting in significant differences in growth factor protein concentrations. Additionally, both sensory and motor axons were found to regenerate most effectively when they were paired with modality-specific Schwann cells. We thought it unlikely, however, that Schwann cells accompanying all afferent or all efferent axons would be identical in their growth factor expression. Variables such as the difference in regeneration vigor between dorsal root and peripheral nerve (Wujek and Lasek, 1983; Oblinger and Lasek, 1984) and the multiplicity of growth factor expression patterns within the DRG (Lawson, 2005) suggested that Schwann cell phenotype might be more varied.
In the current experiments, we used real-time RT-PCR to examine the expression of 11 growth factors 5 days, 15 days, and 30 days after denervation of dorsal root, cutaneous nerve, cutaneous unmyelinated axons, muscle nerve afferents, muscle nerve efferents, and ventral root. In preparations in which a sub-population of axons was denervated (unmyelinated axons within cutaneous nerve, and afferent or efferent axons within muscle nerve), Schwann cells of the remaining axons continued to function normally and presumably expressed growth factors at baseline levels. We found that growth factor expression by denervated Schwann cells was not only subject to further regulation within the previously- defined afferent and efferent groups, but also varied along a central-peripheral axis. The traditional view of myelinating Schwann cells as a homogenous population is thus modified by the realization that complex regulation produces a wide variety of Schwann cell phenotypes. As a consequence, Schwann cells are likely to vary in their ability to support regeneration of transected axons. Additionally, we found that Schwann cell phenotype is maintained for 2 weeks in vitro without instructive cues from axons or basal lamina.
In our previous work, all Schwann cells within ventral root or cutaneous nerve grafts were denervated at the time of graft harvest and thus underwent Wallerian degeneration simultaneously (Hoke et al, 2006), so it was possible to evaluate their support for regeneration as an homogenous population, and thus to learn that support for regeneration was modality-specific. It is not possible, however, to assess the support provided for regeneration by the subsets of Schwann cells denervated in the current experiments. Were a partially denervated nerve harvested as graft, the remaining, previously innervated Schwann cells would be denervated as well, masking the effect of the selective denervation. As a result, the current experiments do not include a regeneration component.
MATERIALS AND METHODS
Surgical Preparations
Female Lewis rats were anaesthetized by intramuscular injection of ketamine (87 mg/Kg) and xylazine (13 mg/Kg). All procedures were performed under sterile conditions and were approved by the Johns Hopkins Animal Care and Use Committee. Six experimental configurations were generated to denervate the Schwann cells that accompany specific sub-populations of axons: dorsal root, cutaneous nerve, cutaneous unmyelinated axons, muscle nerve afferents, muscle nerve efferents, and ventral root (Figure 1). Surgery was limited to the femoral nerve system, which consists of the L2, L3, and L4 dorsal and ventral roots, the femoral nerve trunk, and the muscle (quadriceps) and cutaneous (saphenous) femoral branches.
Figure 1.
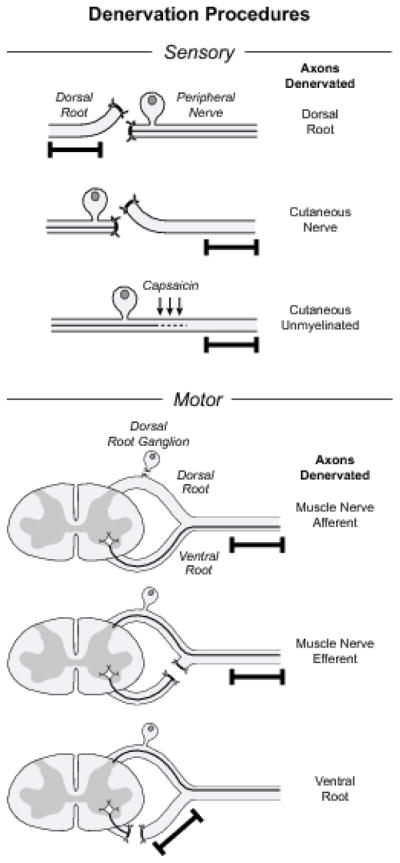
Procedures used to denervate specific Schwann cell populations for PCR analysis in these experiments. The segment of nerve removed for study is indicated by the black bar. The cutaneous branch of the rat femoral nerve was denervated by proximal ligation and interruption of the femoral nerve trunk. Exposure of dorsal roots, DRGs, and ventral root fibers contributing to the femoral nerve was accomplished through unilateral laminectomies at the L2, L3, and L4 levels. Dorsal root was denervated by interrupting it just proximal to the ganglia, afferents in the femoral muscle branch by excising the exposed DRGs, and efferents in the femoral muscle branch by interrupting the ventral root while preserving the dorsal root and DRG. Although the dorsal and ventral roots are shown as separate structures for diagrammatic clarity, the dorsal root ganglion is actually adherent to the ventral root beneath it. Great care is thus required to excise the DRG without injuring the ventral root. Survival of ventral root axons after this preparation has been confirmed in a previous publication from our laboratory (Redett et al., 2005). Ventral root denervation required more proximal laminectomies to expose and interrupt the ventral roots as they exited the spinal cord. Cutaneous unmyelinated axons were denervated by direct application of capsaicin to the femoral nerve.
Schwann cells that accompany dorsal root, muscle nerve afferent, or muscle nerve efferent axons were denervated through lateral laminectomies that exposed the L2, L3, and L4 DRGs and contiguous roots (Figure 1). Dorsal root was ligated with a 10–0 suture near its distal end, and then transected between the suture and the DRG. Muscle nerve afferent Schwann cells in the femoral muscle branch were denervated selectively by excising the L2, L3, and L4 DRGs, and muscle efferent Schwann cells in this nerve were denervated by ligating and transecting the ventral roots while leaving their accompanying DRGs intact. Denervation of ventral root Schwann cells required more proximal laminectomies so that the L2, L3, and L4 ventral roots could be ligated and transected as they exited the spinal cord. In the periphery, Schwann cells in the femoral cutaneous branch were denervated by transecting the parent femoral trunk. Selective denervation of non-myelinating Schwann cells was accomplished by soaking the femoral cutaneous branch in a 1.5% solution of Capsaicin in 20% cyclodextrine for 20 minutes (Mannion et al., 1996).
Electronmicroscopy
Capsaicin-treated nerve was examined to insure the selective denervation of non-myelinating Schwann cells (Figure 2). Nerve specimens were harvested from 250 gm Lewis rats, fixed in 5% gluteraldehyde, osmicated, and embedded in Epon-Araldite. Thin sections were examined with a Hitachi H-600 electron microscope at 1,000–40,000x and non-myelinating Schwann cells were identified and scored as to their compliment of unmyelinated axons.
Figure 2.
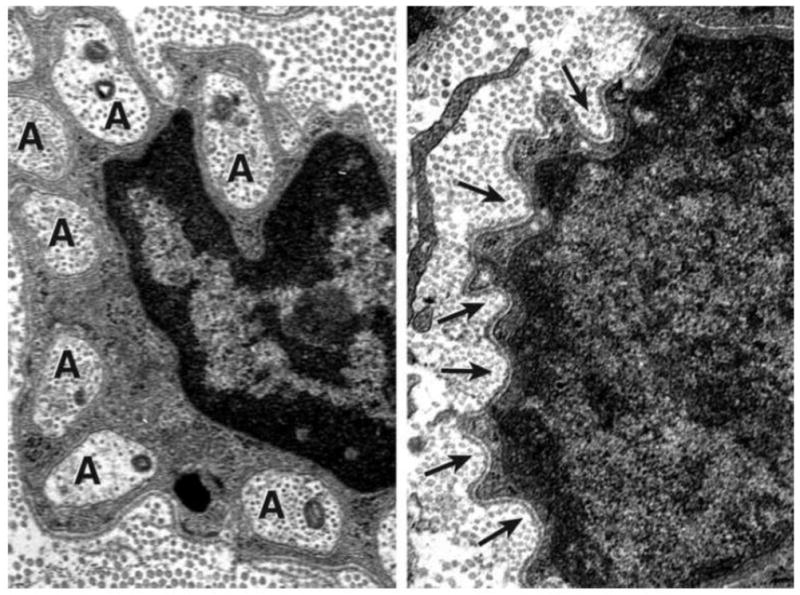
Electron micrograph, original magnification 40,000x. Left. Intact Remak bundle with multiple unmyelinated axons (A) surrounding the nucleus of the parent Schwann cell. Right. After treatment with capsaicin, empty pockets (arrows) mark the sites previously occupied by unmyelinated axons.
PCR Experiments
Peripheral nerve and root segments denervated by each of the 6 surgical preparations were examined at 5 days, 15 days, and 30 days after surgery, yielding a total of 18 experimental groups. Ten nerves or roots were denervated in each group, requiring a total of 180 surgical preparations for PCR analysis. The 10 nerves or roots that represented each experimental condition were combined into 3 or 4 pools depending upon their mRNA content, based on previous experience, yielding an n of 3 or 4 for each PCR analysis. Additional normal femoral nerves and roots were used for normalization of the mRNA levels (i.e. day 0).
Total RNA was extracted from the nerves using Trizol (Invitrogen). The cDNA was synthesized using 2 μg of total RNA in the presence of Ready-to Go™ You Prime First Strand beads (Amersham) and random primers (Invitrogen/Life Technologies). Measurements of mRNA levels were performed by real-time RT-PCR using the two-color DNA Engine Opticon System (M.J. Research Inc.) and the relative amount of gene of interest was normalized to the mRNA amount of an internal control (GAPDH) in the same PCR reaction. Expression of GAPDH itself was found to increase slightly in denervated nerve, but only 1–2 fold at most. This would tend to minimize our assessment of growth factor upregulation rather than accentuate it. To avoid the possibility of amplifying contaminating DNA, all of the primers for real-time RT-PCR were designed with an intron sequence inside the cDNA to be amplified; reactions were performed with appropriate negative control samples (template-free control samples); a uniform amplification of the products was rechecked by analyzing the melting curves of the amplified products (dissociation graphs); the melting temperature (Tm) was 57°C to 60°C; the probe Tm was at least 10°C higher than primer Tm; and gel electrophoresis was performed to confirm the correct size of the amplification and the absence of nonspecific bands. Protein products were sequenced and the sequences were validated at the time each probe was constructed. The RT-PCR experiments were conducted by a technician who was blinded to the nature of groups. The Real-time PCR parameters and the primer and probe sequences have been published (Hoke et al., 2006). The data was analyzed using analysis of variance with post-hoc correction for multiple comparisons (The critical a level was set at p=0.005).
Protein isolation and ELISA
Protein measurements of NGF, GDNF and BDNF were made using kits (catalogue numbers G7630, 7620 and 7611) from Promega according to the manufacturer’s instructions. Optical density was read using SPECTRAmax340PC and concentrations were calculated based on the standards curve.
Schwann cell culture
Dorsal and ventral roots were dissected from decapitated postnatal day 10 rats and enzymatically dissociated with 0.25% trypsin in L-15 medium (Gibco, Catalog #11415). On the second day of culture 5μM of Ara-C (cytosine 1-beta-D-arabinofuranoside; Sigma-C, Catalog #1768) was added to the cultures to eliminate dividing fibroblasts. Schwann cells were maintained in 6-well plates with DMEM media (Gibco 11965-092) containing 10% fetal bovine serum, penicillin (1U/L), streptomycin (1U/L), 1mg/mL pituitary extract (Sigma, Catalog # P1167), and 20ng/mL Neuregulin (R&D Systems, Catalog # 396-HB). Cells or cell supernatant were collected for mRNA isolation and ELISA experiments respectively, when cells were 90–95% confluent.
RESULTS
Differences in growth factor expression among normal, uninjured nerves
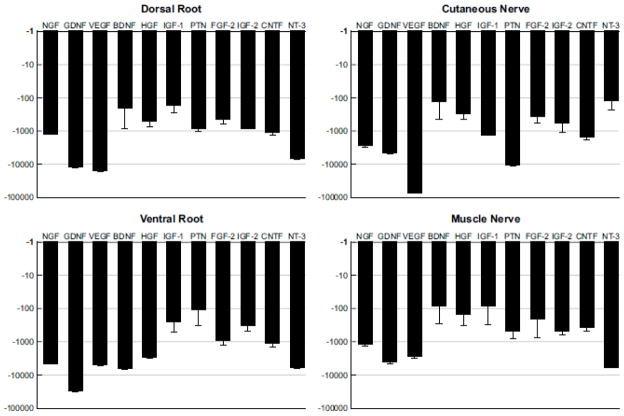
We used real-time RT-PCR to quantify the expression of eleven growth factors in each of the Schwann cell populations evaluated in these experiments. These are the same factors studied in previous work that was limited to ventral root and cutaneous nerve (Hoke et al., 2006), and were chosen at that time based on our assessment of their potential for differential expression in motor and sensory Schwann cell populations. We began by quantifying the baseline expression of these factors in the four types of nerve that provide the anatomical substrate for the selective denervation procedures studied here: dorsal root, ventral root, cutaneous nerve, and muscle nerve (Figure 1). This information, derived entirely from normal, uninjured nerve, forms the basis for comparing subsequent upregulation of these factors after modality- or location-specific Schwann cell denervation. To provide a uniform basis for comparison, the relative expression of each factor is compared to that of a housekeeping gene, GAPDH, expressed as the fold-difference in expression of the two (Figure 3). As GAPDH expression is substantially greater than growth factor expression in resting nerve, all values are negative.
Figure 3.
Baseline expression of growth factors in the normal, uninjured nerves that will serve as the anatomical substrate for denervation experiments: dorsal root, ventral root, cutaneous nerve, and muscle nerve. Expression of each growth factor is measured relative to that of GAPDH, and is graphed as the fold difference between the two on a logarithmic scale. Since GAPDH is expressed in resting Schwann cells at much higher levels than are growth factors, all values are negative.
In resting dorsal root, IGF-1 and BDNF were expressed most prominently; the majority of factors lay in the mid-range, but NT-3, VEGF and GDNF were expressed at relatively low levels. Some aspects of this pattern were also seen in ventral root, with relatively high baseline expression of IGF-1 and relatively low expression of GDNF and VEGF. Ventral root was most prominently characterized, however, by high expression of PTN. Cutaneous nerve, in contrast, had very low PTN expression and relatively high expression of NT-3, BDNF, HGF, and FGF-2. In muscle nerve, PTN expression resembled that of ventral root, and was far greater than that in cutaneous nerve. IGF-1 and BDNF were also expressed at relatively high levels, while NT-3, GDNF and VEGF were expressed at relatively low levels.
Denervation of non-myelinating Schwann cells with capsaicin
Capsaicin-treated cutaneous nerves were examined by electron microscopy at 1,000x to 40,000x magnification and non-myelinating Schwann cells were identified and scored as to their denervation. In the 12 nerves examined, a mean of 52% of non-myelinating Schwann cells were denervated completely, and an additional 32% had only one axon remaining, confirming the effectiveness of capsaicin denervation.
Differences in growth factor expression after denervation
To search for heterogeneity amongst populations of denervated Schwann cells, we used real-time RT-PCR to examine the expression of our panel of 11 growth factors 5 days, 15 days, and 30 days after Schwann cell denervation. Whereas we were limited to determining baseline growth factor expression in four types of nerve presented by normal anatomy - dorsal root, ventral root, cutaneous nerve, and muscle nerve (Figure 3) - anatomically selective denervation permitted individual evaluation of growth factor upregulation by six distinct sub-populations of denervated Schwann cells: dorsal root, cutaneous nerve, cutaneous unmyelinated axons, muscle nerve afferents, muscle nerve efferents, and ventral root. (Figures 4 and 5, and Table 1). When comparing expression levels, significance was placed at the p=0.005 level.
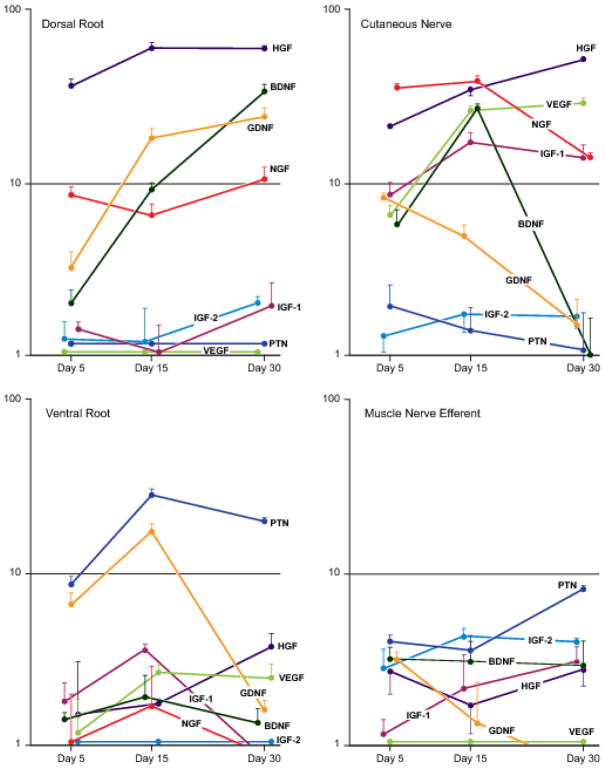
Figure 4.
Expression patterns of selected growth factor genes in dorsal root, cutaneous nerve, ventral root, and muscle nerve efferents that have been denervated for 5, 15, or 30 days. Expression is graphed as the fold-increase in mRNA in relation to baseline expression levels (Figure 3) on a logarithmic scale. Factors that are minimally upregulated are shown along the baseline for purposes of comparison. Raw data are presented in Table I.
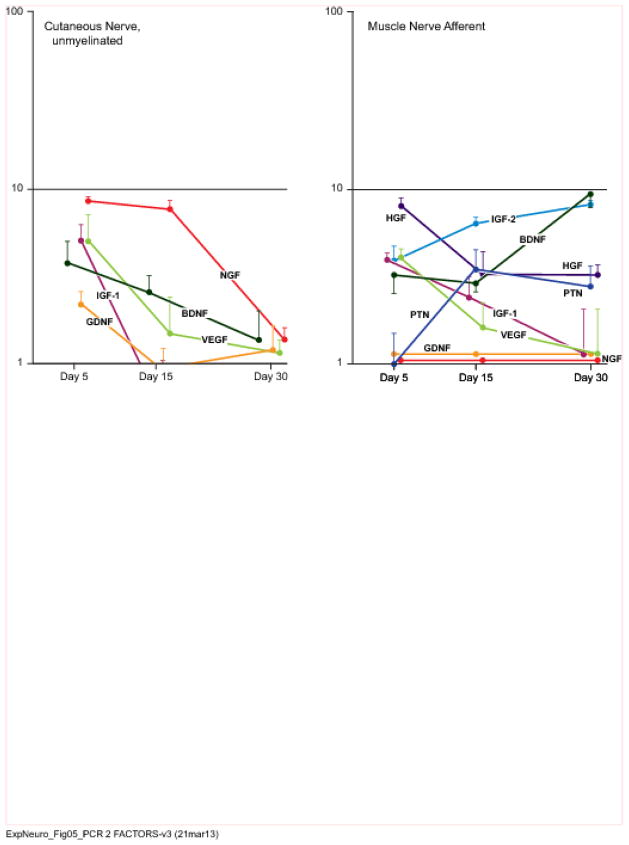
Figure 5.
Expression patterns of selected genes by muscle nerve afferents and cutaneous unmyelinated fibers that have been denervated for 5, 15, or 30 days. Expression is graphed as the fold-increase in mRNA in relation to baseline expression levels (Figure 3) on a logarithmic scale. Factors that are minimally upregulated are shown along the baseline for purposes of comparison. Raw data are presented in Table I.
Table I.
Expression of eleven growth factors by the six sub-populations of denervated Schwann cells studied. Expression levels of each growth factor are quantified as the fold increase in expression compared to baseline expression of that factor in normal, uninjured nerve.
| Dorsal Root | Cutaneous Nerve | Ventral Root | Muscle Nerve Efferent | Muscle Nerve Afferent | Cutaneous Nerve, unmyelinated | |
|---|---|---|---|---|---|---|
| NGF | ||||||
|
|
||||||
| Day 5 | 8.57 (9.5) | 35.71 (2.12) | 1.06 (.93) | .47 (.19) | 1.76 (.28) | 8.40 (.49) |
| Day 15 | 6.50 (1.13) | 38.59 (3.01) | 1.70 (1.16) | .26 (.53) | .15 (.43) | 7.62 (.81) |
| Day 30 | 10.56 (1.82) | 13.92 (1.14) | .85 (.78) | .60 (.27) | −1.12 (.71) | 1.38 (.22) |
| BDNF | ||||||
|
|
||||||
| Day 5 | 2.00 (.41) | 5.69 (1.29) | 1.42 (.15) | 3.20 (.51) | 3.22 (.82) | 3.73 (1.17) |
| Day 15 | 9.19 (.84) | 26.72 (2.02) | 1.93 (.64) | 3.07 (.94) | 2.86 (.34) | 2.57 (.58) |
| Day 30 | 34.29 (2.96) | 1.01 (.65) | 1.36 (.29) | 2.91 (1.12) | 9.20 (1.63) | 1.37 (.63) |
| GDNF | ||||||
|
|
||||||
| Day 5 | 3.25 (.76) | 8.18 (.49) | 6.49 (1.13) | 3.13 (.34) | 2.07 (.68) | 2.14 (.38) |
| Day 15 | 18.38 (2.31) | 4.92 (.76) | 17.15 (1.86) | 1.36 (.95) | .50 (.19) | .93 (.29) |
| Day 30 | 24.25 (3.09) | 1.51 (.61) | 1.62 (.22) | .44 (.46) | .82 (1.14) | 1.19 (.45) |
| PTN | ||||||
|
|
||||||
| Day 5 | .77 (.38) | 1.95 (.63) | 8.57 (.93) | 4.00 (1.21) | 1.00 (.49) | 159 (.35) |
| Day 15 | 1.00 (.40) | 1.41 (.49) | 27.85 (2.16) | 3.56 (.65) | 3.46 (.99) | 1.01 (.28) |
| Day 30 | 1.07 (.62) | 1.07 (.71) | 19.69 (1.01) | 8.06 (2.13) | 2.77 (.83) | .74 (.44) |
| IGF-1 | ||||||
|
|
||||||
| Day 5 | 1.41 (.16) | 8.57 (1.49) | 1.79 (.53) | 1.18 (.24) | 3.86 (.38) | 5.06 (1.12) |
| Day 15 | 1.05 (.46) | 17.15 (2.37) | 3.60 (.28) | 2.14 (1.21) | 2.41 (.72) | .59 (.45) |
| Day 30 | 1.95 (.71) | 13.93 (2.71) | .92 (.89) | 3.05 (.67) | 1.16 (.89) | .46 (.33) |
| IGF-2 | ||||||
|
|
||||||
| Day 5 | 1.26 (.34) | 1.31 (.31) | 1.07 (.29) | 2.83 (.62) | 3.83 (.83) | 1.25 (.44) |
| Day 15 | 1.20 (.69) | 1.74 (.49) | .94 (.83) | 4.28 (.49) | 6.27 (.55) | .39 (.51) |
| Day 30 | 2.00 (.22) | 1.68 (.28) | 1.00 (.42) | 4.00 (.18) | 8.00 (.49) | .75 (.18) |
| CNTF | ||||||
|
|
||||||
| Day 5 | −3.28 (−.26) | .56 (.68) | −1.42 (−.39) | −.98 (−.35) | .87 (.16) | .26 (.58) |
| Day 15 | −4.27 (−.75) | −1.14 (−.83) | −2.03 (−1.12) | −.81 (−.68) | −1.75 (−.82) | −2.55 (−1.12) |
| Day 30 | −4.96 (−1.16) | .58 (.28) | −1.63 (−.81) | .48 (.71) | −1.88 (−.66) | −1.89 (−.62) |
| VEGF | ||||||
|
|
||||||
| Day 5 | .23 (.69) | 6.49 (.93) | 1.19 (.68) | 1.46 (.35) | 4.00 (.53) | 4.99 (2.02) |
| Day 15 | .71 (1.15) | 25.99 (1.89) | 2.67 (.26) | .26 (.79) | 1.62 (.62) | 1.48 (.89) |
| Day 30 | .93 (.49) | 28.84 (2.01) | 2.46 (.49) | .26 (.29) | 1.15 (.21) | 1.15 (.21) |
| HGF | ||||||
|
|
||||||
| Day 5 | 37.05 (2.90) | 21.11 (.28) | 1.51 (1.56) | 2.71 (.92) | 7.89 (.84) | 2.75 (.35) |
| Day 15 | 60.82 (4.16) | 34.29 (4.02) | 1.76 (.12) | 1.72 (.76) | 3.22 (1.12) | 3.60 (1.18) |
| Day 30 | 60.41 (1.98) | 51.95 (1.93) | 3.73 (.73) | 2.73 (.68) | 3.20 (.46) | 1.13 (.66) |
| FGF-2 | ||||||
|
|
||||||
| Day 5 | .47 (.51) | −1.18 (−.51) | −1.44 (−1.01) | .97 (.26) | .96 (.62) | −1.26 (−.62) |
| Day 15 | .14 (.38) | 1.29 (.21) | −1.00 (−.59) | .26 (.68) | −1.05 (−.44) | .38 (.56) |
| Day 30 | .54 (.61) | 1.27 (.34) | .71 (.43) | .83 (.49) | −.85 (−.55) | −1.60 (−.89) |
| NT-3 | ||||||
|
|
||||||
| Day 5 | 4.08 (.82) | .95 (.62) | 1.11 (.92) | 1.00 (.62) | 1.39 (.18) | −1.58 (−.36) |
| Day 15 | 2.34 (.32) | −1.43 (−.44) | 1.55 (.36) | 1.41 (.19) | 2.46 (.45) | −1.46 (−.69) |
| Day 30 | 1.80 (.45) | 1.17 (.55) | −.84 (−.41) | 3.92 (.72) | 1.15 (.56) | −1.66 (−.86) |
More growth factors were upregulated in excess of 10-fold by denervated cutaneous nerve than by any of the other Schwann cell populations. The most prominent of these was HGF, which was expressed at similarly dramatic levels by cutaneous nerve and dorsal root. Additionally, HGF was upregulated by ventral root, muscle efferent pathways, and muscle afferent pathways, but always to a significantly lower degree than that in cutaneous nerve. NGF was also upregulated at high levels by cutaneous nerve and dorsal root. Peripheral expression of NGF peaked at 15 days, while that in the dorsal root rose more slowly, peaking at 30 days. Non-myelinating Schwann cells contributed to total NGF expression within cutaneous nerve at both 5 and 15 days. NGF expression by ventral root and muscle nerve efferents was negligible. VEGF expression was similar to that of HGF and NGF within cutaneous nerve at 15 days, and intermediate between the two at 30 days, with significant upregulation by non-myelinating Schwann cells at 5 days. VEGF was also expressed by muscle nerve afferents; since baseline expression was much higher in muscle nerve than in cutaneous nerve (Figure 3), the apparent differences between the two at 15 and 30 days in Figures 4 and 5 are not significant. In stark contrast, dorsal root and ventral root upregulated VEGF to a minimal degree.
BDNF and IGF-1 complete the roster of factors upregulated more than 10-fold by denervated cutaneous nerve. BDNF was expressed vigorously by cutaneous nerve and dorsal root, but with strikingly different patterns; expression peaked at 15 days in cutaneous nerve, dropping off to insignificant levels by 30 days, while it continued to rise throughout the observation period in dorsal root. BDNF expression was significantly higher in dorsal root than in ventral root at all time periods, a difference magnified by the relatively high baseline expression of BDNF in cutaneous nerve (Figure 3). Expression by muscle nerve afferents was significantly lower than that by cutaneous nerve at 15 days, but was significantly higher at 30 days once cutaneous nerve expression had dropped precipitously. IGF-1 was upregulated prominently by cutaneous nerve, maintaining a high level of expression from 15 to 30 days. IGF-1 was also expressed to a very modest degree by muscle afferents and by non-myelinating Schwann cells, but only minimally by central sensory projections within the dorsal root or in the ventral root.
Functionally disparate Schwann cell populations, those in dorsal and ventral root, were united by their dramatic upregulation of GDNF. The increase from 5 to 15 days was similar in both nerves, but expression was significantly different at 30 days because of a sharp drop-off in ventral root. GDNF was also expressed by cutaneous nerve, but at significantly lower levels than those seen in dorsal root at both 15 and 30 days, and by motor efferents at significantly lower levels than those in cutaneous nerve at 15 and 30 days.
Pleitrophin emerged as the dominant factor within the motor system. It had the highest baseline expression in uninjured ventral root (Figure 3), and then experienced dramatic upregulation in response to denervation. PTN was also the most highly upregulated factor by muscle nerve efferents, though to a significantly lower degree than in ventral root at all time periods. Upregulation by denervated muscle nerve afferents was modest, and by dorsal root and cutaneous nerve was negligible. IGF-2 was upregulated to a moderate degree by both afferents and efferents within muscle nerve.
Based upon the above findings, the peak growth factor upregulation by several populations of Schwann cells appears to be regulated to varying degrees by the modality of their associated axons and by their central-peripheral location: cutaneous afferent, distal- IGF-1 and VEGF; cutaneous afferent, proximal and distal- NGF, BDNF, HGF; afferent and efferent, proximal- GDNF; muscle efferent, proximal and distal-PTN; muscle afferent and efferent, distal- IGF-2.
Growth factor protein levels
ELISA for NGF, GDNF, and BDNF was performed on several pertinent experimental groups (Figure 6). NGF protein was present at similar concentrations in control cutaneous nerve and dorsal root (CN-138 pg/ml; DR- 107 pg/ml), and its concentration after 15 days of denervation increased in proportion to the NGF upregulation detected by PCR (CN- 238 pg/ml; DR- 136 pg/ml). Resting BDNF concentrations in cutaneous nerve and dorsal root were also similar, but lower (CN- 14 pg/ml; DR- 27 pg/ml). After 30 days of Schwann cell denervation, the low concentration of BDNF in cutaneous nerve is consistent with its downregulation as detected by RT-PCR. The concentration in dorsal root, however, is less than one might expect given the high relative baseline expression (Figure 3) and subsequent upregulation (Figure 4). Significant amounts of BDNF might therefore be subject to local consumption or transport elsewhere. Alternatively, BDNF expression may be regulated more tightly at the protein level so that less BDNF mRNA is translated.
Figure 6.
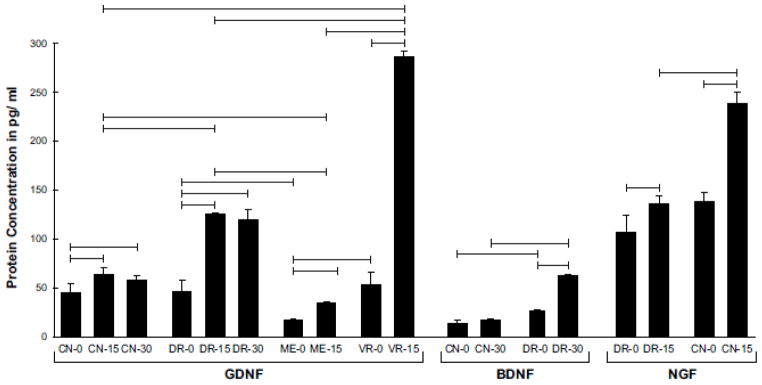
Concentrations of GDNF, BDNF, and NGF protein in normal cutaneous nerve, dorsal root, ventral root, and muscle nerve, and in selected populations of denervated Schwann cells. Horizontal bars linking different groups indicate a significant difference in concentration (p< 0.05). GDNF was upregulated prominently by DR and VR, and the resulting protein concentrations were significantly higher in these structures than in their peripheral extensions, CN and ME. The BDNF profile is consistent with the PCR findings after 30 days of denervation, with expression in CN returning to baseline and that in DR peaking. The increased pace of NGF upregulation in CN when compared with DR was also reflected in protein concentrations.
GDNF concentrations increased significantly with denervation in four pathway sub-types. Increases were modest in cutaneous nerve and muscle efferents, areas in which there were statistically significant though relatively minor increases in gene expression (Figures 4 and 5) (CN-0: 45 pg/ml; CN-15: 64 pg/ml; CN- 30: 58 pg/ml; ME-0: 17 pg/ml; ME-15: 35 pg/ml). In dorsal root, more substantial increases were seen at both 15 and 30 days that again paralleled increases in gene expression (DR-0: 46 pg/ml; DR-15: 125 pg/ml; DR-30:120 pg/ml). The most dramatic changes seen were in the ventral root (VR-0: 53 pg/ml; VR-15: 286 pg/ml). Overall, protein concentrations were consistent with levels of gene expression, with the greatest variability exemplified by the relatively low levels of BDNF and high levels of GDNF resulting from similar degrees of gene upregulation.
Schwann cell culture
The persistence of growth factor expression for 15–30 days after denervation indicates that Schwann cells maintain their growth factor expression phenotype in spite of the cell divisions that accompany Wallerian degeneration. When confronted with the more severe challenge of prolonged reinnervation by inappropriate axons, Schwann cell gene expression maintains its essential character, yet gradually shifts towards that of the reinnervating axons (Hoke et al., 2006). Multiple replications in tissue culture without the potentially instructive influence of native basal lamina present an even greater challenge. Expression of NGF, GDNF, BDNF, and PTN was measured with competitive RT-PCR in fresh dorsal and ventral roots from 10-day-old rats, and in Schwann cells cultured from these sources for 2 weeks (Figure 7). Expression of mRNA for BDNF was higher by 60-fold in dorsal root, and expression of mRNA for PTN was higher by 149-fold in ventral root. These differences are less pronounced than those observed in the tissues of adult rats (Figure 3) After two weeks in vitro the magnitude of the difference had diminished, yet the proportions were similar, with a 26-fold higher expression for BDNF in Schwann cells from dorsal root and 49-fold for PTN in Schwann cells from ventral root. However, this difference in expression level was not maintained over time as by 4 weeks in vitro levels of all 4 neurotrophic factor mRNAs were similar in Schwann cell cultures from both ventral and dorsal roots. ELISA for BDNF was performed on the cultured cells; at 2 weeks the concentration of BDNF was significantly higher in dorsal root Schwann cells (DR cells: 36 pg/ml; VR cells 20 pg/ml), but by 3 weeks BDNF concentrations had equalized in the two populations (DR cells 59 pg/ml; VR cells 58 pg/ml). Schwann cells from dorsal and ventral root are thus able to maintain elements of their characteristic phenotypes in the absence of external cues, but they do not maintain their phenotype indefinitely, a finding with clear implications for the use of Schwann cell transplants to promote regeneration.
Figure 7.
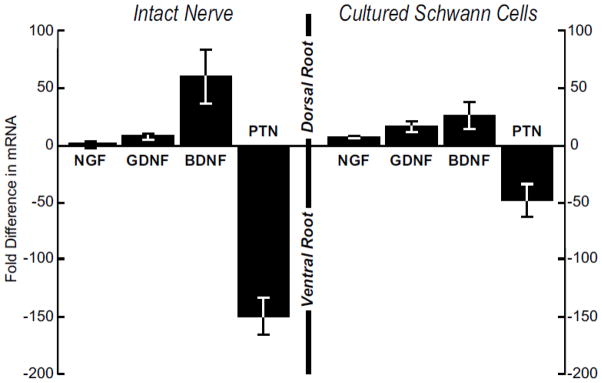
Comparison of the expression of NGF, GDNF, BDNF, and PTN by intact nerve and by Schwann cells cultured from 10-day-old rats. Expression by dorsal root and dorsal root Schwann cells is graphed above the line, and that by ventral root or ventral root Schwann cells is graphed below the line. The expression pattern seen in intact nerve is mirrored by Schwann cells that have been culture for 2 weeks, indicating that Schwann cell phenotype persists through several divisions in the absence of instructive signals from axons or basal lamina.
DISCUSSION
Experimental Model
Since the discovery of NGF expression by Schwann cells (Rush, 1984), peripheral nerve has been identified as the source of several growth factors (reviewed in Brushart, 2011). Most of these investigations have involved the rodent sciatic nerve (eg. Funakoshi et al., 1993; Henderson et al., 1994), an admixture of many axon types. While the DRG, spinal cord, and muscle have also been investigated, the dorsal and ventral roots have largely been ignored. Similarly, growth factor expression by subsets of Schwann cells within mixed nerve has not been explored. Redett et al. (2005) recently introduced the technique of excising DRGs serving the rat femoral nerve so that only motor axons would regenerate after a femoral nerve lesion. At that time, we demonstrated that, with careful microsurgical technique, DRGs could be separated from the ventral roots to which they adhere with minimal damage to underlying motor axons. We here repurpose this operation to selectively denervate the Schwann cells that accompany afferent axons within muscle nerve while leaving motor efferent axons intact. Additionally, we have developed surgical techniques to denervate ventral root by transecting it as it exits the spinal cord, and to denervate muscle efferent axons within peripheral nerve by transecting ventral root just proximal to the DRG. Considerable effort was required to develop these procedures in such a way as to minimize blood loss and damage to adjacent neural structures. Non-myelinating Schwann cells within cutaneous nerve, in contrast, were chemically denervated by treating the nerve with capsaicin, a procedure previously shown to eliminate the axons contributing to these pathways (Mannion et al., 1996). With this armamentarium of procedures, we can now examine growth factor production by several subsets of Schwann cells that have not been isolated previously in vivo.
Schwann cell phenotype
Mature Schwann cells are thought to express one of two phenotypes, myelinating or nonmyelinating (Jessen and Mirsky, 2002), and these have been shown to differ in their upregulation of BDNF in response to denervation (Friedman et al., 1996). Recently, we found that cutaneous nerve and ventral root, predominately myelinated axon populations, also differ in their growth factor expression (Hoke et al., 2006). This phenotypic variability was not detected previously because growth factor production was routinely investigated in mixed nerve rather than in modality-specific axon sub-populations (Funakoshi et al., 1993; Henderson et al., 1994). The majority of growth factor in peripheral nerve emanates from Schwann cells (eg Hammarberg et al., 1996; Meyer et al., 1992), although macrophages and fibroblasts may also contribute (Heumann et al., 1987; Cheng et al., 1996). It is possible that expression differences between roots and peripheral nerve could be influenced by differential ingrowth of macrophages, but extremely unlikely that sub-populations within a given nerve, such as cutaneous and motor axons in the femoral trunk, could be subject to this type of differential influence.
The profiles of growth factor expression by denervated Schwann cells were found to vary in magnitude and pattern on the basis of both central-peripheral location and the modality of their associated axons. The most striking findings of this study involved differences in the magnitude of expression between afferent and efferent pathways. Both HGF and BDNF were elevated in both central and peripheral afferents: dorsal root, cutaneous nerve, and muscle afferents, and were elevated to significantly lower degrees in both central and peripheral efferents: ventral root and muscle efferents. NGF expression followed a similar pattern, except that cutaneous unmyelinated axons made a significant contribution and little upregulation was noted in muscle afferents. High levels of VEGF and IGF-1 expression were limited to the periphery, where they were significantly greater in cutaneous nerve than in muscle efferents. Conversely, Pleiotrophin was elevated dramatically in ventral root and to a lesser degree in muscle efferents, but very little in dorsal root or cutaneous nerve. Given its high baseline expression in ventral root, Pleiotrophin emerged as the most characteristic motor factor. This finding is consistent with identification of Pleiotrophin as a motor specific neurotrophic factor (Mi et. al., 2007).
The magnitude of growth factor expression was also influenced by central-peripheral location. This is demonstrated most prominently by GDNF, whose expression levels were significantly greater at 15 and 30 days centrally in dorsal and ventral roots than in their respective peripheral components, cutaneous nerve and muscle efferents. Although baseline GDNF expression was minimal, protein levels in denervated nerve were substantial and corresponded in magnitude to the degree of gene upregulation in each instance. BDNF, in contrast, had higher baseline expression and was upregulated to a similar degree, but resulting concentrations of BDNF protein were substantially lower. Within the sensory system, IGF-1 was expressed at significantly higher levels in denervated cutaneous nerve than in denervated dorsal root at all time periods.
Location along the neuraxis also influenced the patterns of growth factor expression. This was illustrated most prominently by GDNF and BDNF. The expression of both in dorsal root increased throughout the period of observation, reaching very high levels by 30 days. In cutaneous nerve, in contrast, BDNF expression followed a similar trajectory from day 5 to day 15; then decreased precipitously, and GDNF decreased over both intervals. Regeneration proceeds more rapidly in the periphery than in dorsal root (Komiya, 1981), so it is possible that regenerating axons are contacting Schwann cells and downregulating growth factor production more rapidly in cutaneous nerve. Enhancing the regeneration state of the DRG neuron with a conditioning lesion or the application of cAMP is known to enhance the regeneration state of these neurons (Richardson and Issa, 1984; Neumann et al., 2002); it will be interesting to see if these maneuvers also modify the pattern of growth factor production by the dorsal root towards that in the periphery.
In these experiments, the patterns of growth factor expression by denervated Schwann cells are maintained through multiple cell divisions over several weeks. In previous work, we determined that the growth factor phenotype of Schwann cells persists to a significant degree even when the Schwann cells have been reinnervated with modality-inappropriate axons (Hoke et al., 2006). To pose an even more stringent test of phenotypic durability, we maintained Schwann cells in vitro through several cell divisions. Under these conditions, Schwann cells derived from dorsal or ventral root retained characteristic sensory and motor expression patterns for 2 weeks (Figure 7). As Schwann cells divide every 2–3 days in vitro, phenotypic identity must survive at least 4–5 cell divisions without cues from either axons or basal lamina. When rat Schwann cells are cultured for longer than 2 weeks their sensory/motor identity becomes less distinct, as shown by our data and as recently described by Jesuraj et al. (2012).
The mechanism that generates diversity of Schwann cell phenotype has yet to be identified. Recently, however, the transcription factor c-Jun has emerged as a primary modulator of the repair program in Schwann cells (Arthur-Farraj et al., 2012; Fontana et al., 2012). c-Jun can be activated through MAPK signaling pathways, which have been identified as regulators of Schwann cell differentiation (Napoli et al., 2012; Yang et al., 2012), and in turn promotes expression of several components of the repair system including GDNF and BDNF (Arthur-Farraj et al., 2012; Fontana et al., 2012). The impact of manipulating individual signaling pathways on growth factor production by Schwann cells is currently under study in our laboratory.
Implications
The current findings help resolve apparent discrepancies among recent examinations of pathway-specific regeneration. In initial experiments, Hoke at al. (2006) compared regeneration of pure populations of sensory neurons to regeneration of pure populations of motor neurons. These defined axon populations were used to reinnervate grafts of ventral root, in which all myelinated axons are motor, and femoral cutaneous nerve, in which all axons are sensory. Under these conditions, grafts of ventral root preferentially supported motor axon regeneration while grafts of cutaneous nerve preferentially supported sensory regeneration (Hoke et al., 2006). In subsequent work by others, axons from the transected rat tibial nerve and femoral motor branch were challenged with grafts fashioned from the femoral cutaneous and muscle branches and, in both cases, failed to provide evidence for pathway-specific support of regeneration (Neubauer et al., 2010; Kawamura et al., 2010). In these experiments, however, neither the axon sources or the grafts were modality-specific. In both donor nerves, the tibial and the femoral muscle branch, the majority of myelinated axons are afferent rather than motor (Brushart, 1988). Similarly, the “motor” graft was also femoral muscle branch. The current experiments demonstrate that ventral root provides a limited menu of growth factors, whereas femoral muscle nerve expresses a wide variety of factors that have been shown to promote both sensory and motor regeneration. Differential support of motor axon regeneration by ventral root and cutaneous nerve (Hoke et al., 2006) and the absence of differential support by femoral muscle and cutaneous nerves (Neubauer et al., 2010; Kawamura et al., 2010) is thus consistent with the growth factor profile of each nerve.
Our current findings have additional implications. Axons advance as a single front after nerve crush, but regeneration is staggered after nerve transection and repair (Brushart et al., 2002). As axons remain within their original Schwann cell tubes in the former case but rarely do so in the latter (Witzel et al., 2004), it is possible that varying degrees of mismatch between axons and their new Schwann cells will result in proportional modulation of regeneration speed, contributing to staggered regeneration. This possibility highlights the need for definition of the precise contribution of individual Schwann cell-derived growth factors to the regeneration of defined sub-sets of peripheral axons. Once this information is obtained, it should be possible to enhance regeneration specificity by focally increasing the concentration of factors that promote regeneration of a specific axon population, and, no less importantly, to reduce the concentration of confounding factors in inappropriate pathways.
Highlights.
Production of specific growth factors is a manifestation of Schwann cell phenotype
The phenotype of a Schwann cell is linked to the modality of its axonal partner
Within a modality, Schwann cell phenotype may vary by central/peripheral location
Schwann cell phenotype persists for 2 weeks in vitro
Acknowledgments
The authors thank Ms. Kate Weaver for preparation of artwork. Supported by NIH RO1 NS034484 and the Dr. Miriam and Sheldon Adelson Medical Research Foundation.
Abbreviations
- BDNF
brain derived neurotrophic factor
- CNTF
ciliary neurotrophic factor
- FGF-2
fibroblast growth factor 2
- GDNF
glial cell line derived neurotrophic factor
- HGF
hepatocyte growth factor
- IGF-1
insulin-like growth factor 1
- IGF-2
insulin-like growth factor 2
- NGF
nerve growth factor
- NT-3
neurotrophin 3
- PTN
pleiotrophin
- VEGF
vascular endothelial growth factor
Footnotes
None of the authors has a conflict of interest related to this work
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arthur-Farraj P, Latouche M, Wilton D, Quintes S, Chabrol E, Banerjee A, Woodhoo A, Jenkins B, Rahman M, Turmaine M, Wicher G, Mitter R, Greensmith L, Behrens A, Raivich G, Mirsky R, Jessen K. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron. 2012;75:633–647. doi: 10.1016/j.neuron.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TM. Preferential reinnervation of motor nerves by regenerating motor axons. J Neurosci. 1988;8:1026–1031. doi: 10.1523/JNEUROSCI.08-03-01026.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TM, Hoffman PN, Royall RM, Murinson BB, Witzel C, Gordon T. Electrical stimulation promotes motoneuron regeneration without increasing its speed or conditioning the neuron. J Neurosci. 2002;22:6631–6638. doi: 10.1523/JNEUROSCI.22-15-06631.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TM. Motor axons preferentially reinervate motor pathways. J Neurosci. 1993;13:2730–2738. doi: 10.1523/JNEUROSCI.13-06-02730.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TM. Nerve Repair. New York: Oxford university Press; 2011. [Google Scholar]
- Cheng HL, Randolph A, Yee D, Delafontaine P, Tennekoon G, Feldman EL. Characterization of insulin-like growth factor-I and its receptor and binding proteins in transected nerves and cultured Schwann cells. J Neurochem. 1996;66:525–536. doi: 10.1046/j.1471-4159.1996.66020525.x. [DOI] [PubMed] [Google Scholar]
- Fontana X, Hristova M, Da Costa C, Patodia S, Thei L, Makwana M, Spencer-Dene B, Latouche M, Mirsky R, Jessen K, Klein R, Raivich G, Behrens A. c-Jun in Schwann cells promotes axonal regeneration and motoneuron survival via paracrine signaling. J Cell Biol. 2012;198:127–141. doi: 10.1083/jcb.201205025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman H, Jelsma T, Bray G, Aguayo A. A distinct pattern of trophic factor expression in myelin-deficient nerves of Trembler mice: Implications for trophic support by Schwann cells. J Neurosci. 1996;16:5344–5350. doi: 10.1523/JNEUROSCI.16-17-05344.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi H, Frisen J, Barbany G, Timmusk T, Zachrisson O, Verge V, Persson H. Differential expression of mRNAs for neurotrophins and their receptors after axotomy of the sciatic nerve. J Cell Biol. 1993;123:455–465. doi: 10.1083/jcb.123.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarberg H, Piehl F, Cullheim S, Fjell J, Hokfelt T, Fried K. GDNF mRNA in Schwann cells and DRG satellite cells after chronic sciatic nerve injury. Neuroreport. 1996;7:857–860. doi: 10.1097/00001756-199603220-00004. [DOI] [PubMed] [Google Scholar]
- Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M, Simpson LC, Moffet B, Vandlen RA, Koliatsos VE, Rosenthal A. GDNF: A potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994;266:1062–1064. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- Heumann R, Korsching S, Bandtlow C, Thoenen H. Changes of nerve growth factor synthesis in nonneuronal cells in response to sciatic nerve transection. J Cell Biol. 1987;104:1623–1631. doi: 10.1083/jcb.104.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoke A, Redett R, Hameed H, Jari R, Li JB, Griffin JW, Brushart TM. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J Neurosci. 2006;26:9646–9655. doi: 10.1523/JNEUROSCI.1620-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. Signals that determine Schwann cell identity. J Anat. 2002;200:367–375. doi: 10.1046/j.1469-7580.2002.00046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesuraj NJ, Nguyen PK, Wood MD, Moore AM, Borschel GH, Mackinnon SE, Sakiyama-Elbert SE. Differential gene expression in motor and sensory Schwann cells in the rat femoral nerve. J Neurosci Rsch. 2012;90:96–104. doi: 10.1002/jnr.22752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura DH, Johnson PJ, Moore AM, Magill CK, Hunter DA, Ray WZ, Tung TH, Mackinnon SE. Matching of motor-sensory modality in the rodent femoral nerve model shows no enhanced effect on peripheral nerve regeneration. Exp Neurol. 2010;223:496–504. doi: 10.1016/j.expneurol.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiya Y. Axonal regeneration in bifurcating axons of rat dorsal root ganglion cells. Exp Neurol. 1981;73:824–826. doi: 10.1016/0014-4886(81)90215-6. [DOI] [PubMed] [Google Scholar]
- Langley JN, Anderson HK. The union of different kinds of nerve fibers. J Physiol. 1904;31:365–391. doi: 10.1113/jphysiol.1904.sp001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson S. Peripheral Neuropathy. Elsevier/Saunders; Philadelphia: 2005. The peripheral sensory nervous system: Dorsal root ganglion neurons, in; pp. 163–202. [Google Scholar]
- Mannion RJ, Doubell TP, Coggeshall RE, Woolf CJ. Collateral sprouting of uninjured primary afferent A-fibers into the superficial dorsal horn of the adult rat spinal cord after topical capsaicin treatment to the sciatic nerve. J Neurosci. 1996;16:5189–5195. doi: 10.1523/JNEUROSCI.16-16-05189.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Matsuoka I, Wetmore C, Olson L, Thoenen H. Enhanced synthesis of Brain-Derived Neurotrophic Factor in the Lesioned Peripheral Nerve: Different Mechanisms are responsible for the Regulation of BDNF and NGF mRNA. J Cell Biol. 1992;119:45–54. doi: 10.1083/jcb.119.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi R, Chen W, Hoke A. Pleiotrophin is a neurotrophic factor for spinal motor neurons. PNAS. 2007;104:4664–4669. doi: 10.1073/pnas.0603243104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli I, Noon L, Ribeiro S, Kerai A, Parrinello S, Rosenberg L, Collins M, Harrisingh M, White I, Woodhoo A, Lloyd A. A central role for the ERK-signaling pathway in controlling Schwann cell plasticity and peripheral nerve regeneration in vivo. Neuron. 2012;73:729–742. doi: 10.1016/j.neuron.2011.11.031. [DOI] [PubMed] [Google Scholar]
- Neubauer D, Graham JB, Muir D. Nerve grafts with various sensory and motor fiber compositions are equally effective for the repair of a mixed nerve defect. Exp Neurol. 2010;223:203–206. doi: 10.1016/j.expneurol.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Neumann S, Bradke F, Tessier-Lavigne M, Basbaum A. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron. 2002;34:885–893. doi: 10.1016/s0896-6273(02)00702-x. [DOI] [PubMed] [Google Scholar]
- Oblinger MM, Lasek RJ. A conditioning lesion of the peripheral axons of dorsal root ganglion cells accelerates regeneration of only their peripheral axons. J Neurosci. 1984;4:1736–1744. doi: 10.1523/JNEUROSCI.04-07-01736.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon y Cajal S. Degeneration and Regeneration of the Nervous System. London: Oxford University Press; 1928. [Google Scholar]
- Redett R, Jari R, Crawford T, Chen YG, Rohde C, Brushart TM. Peripheral pathways regulate motoneuron collateral dynamics. J Neurosci. 2005;25:9406–9412. doi: 10.1523/JNEUROSCI.3105-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PM, Issa VM. Peripheral nerve injury enhances central regeneration of primary sensory neurones. Nature. 1984;309:791–792. doi: 10.1038/309791a0. [DOI] [PubMed] [Google Scholar]
- Rush RA. Immunohistochemical localization of endogenous nerve growth factor. Nature. 1984;312:364–367. doi: 10.1038/312364a0. [DOI] [PubMed] [Google Scholar]
- Witzel C, Rohde C, Brushart TM. Pathway sampling by regenerating peripheral axons. J Comp Neurol. 2005;485:183–190. doi: 10.1002/cne.20436. [DOI] [PubMed] [Google Scholar]
- Wujek JR, Lasek RJ. Correlation of axonal regeneration and slow component b in two branches of a single axon. J Neurosci. 1983;3:243–251. doi: 10.1523/JNEUROSCI.03-02-00243.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Kim J, Syed N, Tung YJ, Bhaskaran A, Mindos T, Mirsky R, Jessen K, Maurel P, Parkinson D, Kim H. p38 MAPK activation promotes denervated Schwann cell phenotype and functions as a negative regulator of Schwann cell differentiation and myelination. J Neurosci. 2012;32:7158–7168. doi: 10.1523/JNEUROSCI.5812-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]