Figure 3. In vitro transport studies of platinum chemotherapeutics.
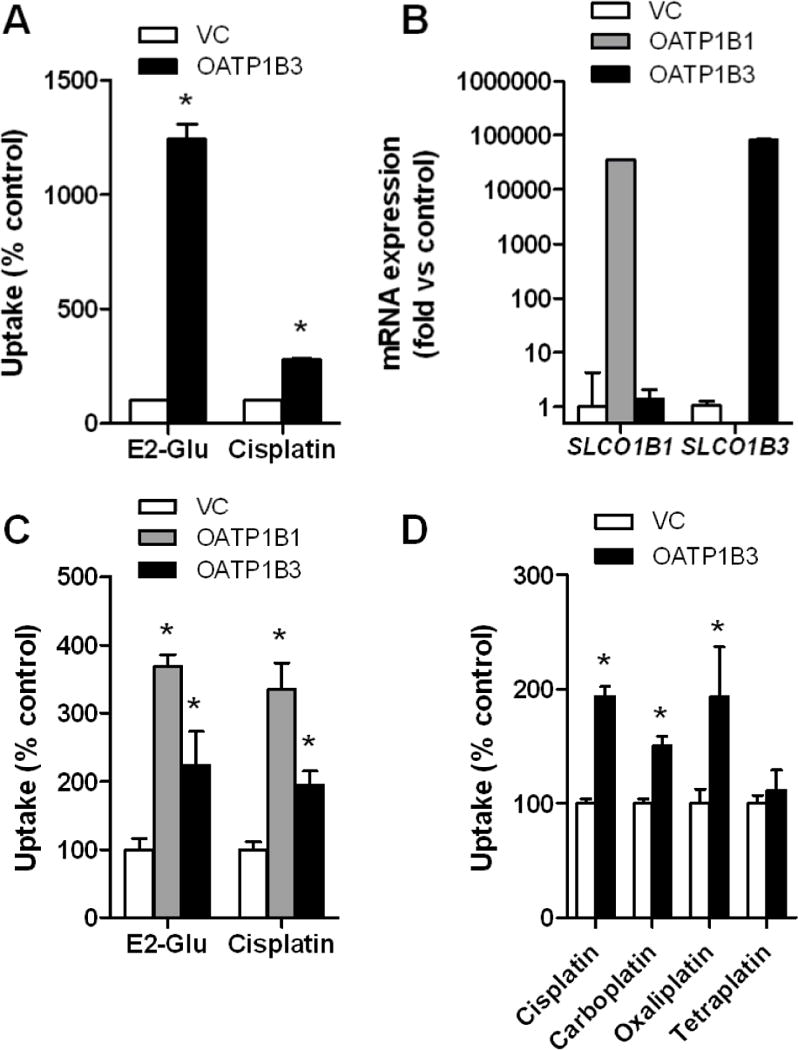
A, Transport of [6,7-3H(N)]estradiol-17β-D-glucuronide (E2-Glu) (2 μM; 60-min incubation), a positive control, and cisplatin (500 μM; 30-min incubation) by human OATP1B3 expressed in Xenopus laevis oocytes. B, mRNA expression of OATP1B1 and OATP1B3 in transfected HEK293 cells. C, Transport of E2-Glu (2 μM; 30-min incubation) and cisplatin (500 μM; 30-min incubation) by human OATP1B1 and OATP1B3 expressed in HEK293 cells. D, Transport of cisplatin, carboplatin, oxaliplatin, and tetraplatin (all 500 μM; 30-min incubation) by human OATP1B3 expressed in HEK293 cells. Data represent the mean of up to 32 observations, and are expressed as the average percent of uptake values (bars) with standard error (error bars) in water-injected cells for oocytes or cells transfected with an empty vector (VC) for HEK293 cells. The star (*) denotes a significant difference from VC (P < 0.05).
