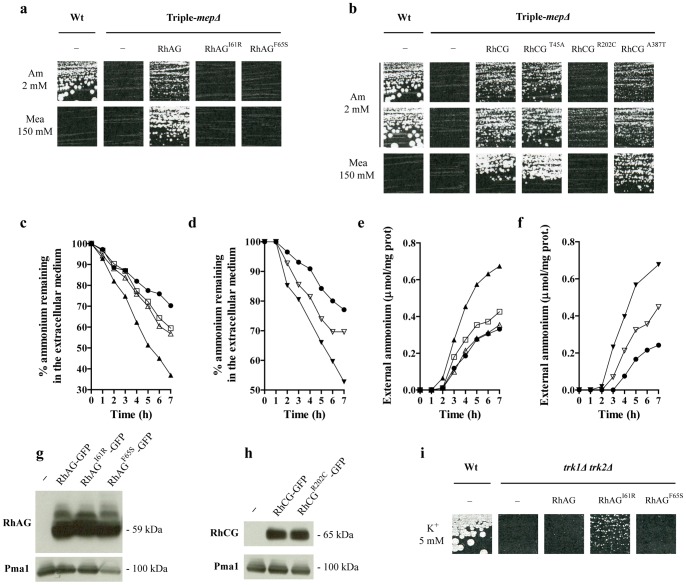
Figure 2. OHSt-related HsRhAG variants and HsRhCGR202C are altered in inherent bidirectional ammonium transport.
a–b Growth tests on solid minimal medium containing as the sole nitrogen source 2 mM ammonium (Am 2 mM), or proline supplemented with a toxic concentration of methylammonium (Mea 150 mM). Wild-type cells (23344c) were transformed with the empty p426 vector (−), and triple-mepΔ cells (31019b) were transformed with the empty p426 vector (−), or with a multi-copy plasmid bearing the native (HsRhAG, HsRhCG) or the mutated HsRHAG and HsRHCG genes (HsRhAGI61R, HsRhAGF65S, HsRhCGT45A, HsRhCGR202C, HsRhCGA387T). Cells were incubated for 7 days (a), and for 7 days (Mea 150 mM, Am 2 mM upper line) or 14 days (Am 2 mM bottom line) (b), at 29°C. c-d Ammonium removal assays. Triple-mepΔ cells (31019b) were transformed with p426 (•), p426-HsRhAG (▴), p426-HsRhAGI61R (Δ), p426-HsRhAGF65S (□), p426-HsRhCG (▾) or p426-HsRhCGR202C (∇). E–f Ammonium excretion assays. Same cells used in (c) and (d). g–h Immunodetection of the HsRhAG-GFP and HsRhCG-GFP variants expressed in yeast. Triple-mepΔ cells (31019b) were transformed with the empty p426 vector (−), or with a multi-copy plasmid bearing GFP-tagged native (HsRhAG-GFP, HsRhCG-GFP) or mutated HsRHAG and HsRHCG genes (HsRhAGI61R-GFP, HsRhAGF65S-GFP, HsRhCGR202C-GFP). Membrane-enriched cell extracts were prepared from cells grown on glutamate minimal medium, separated by SDS-PAGE and immunoblotted with anti-GFP antibodies. ScPma1 was immunodetected as a loading control. i Growth tests of yeast strains on solid YNB medium with glutamine as the sole nitrogen source and supplemented with 5 mM KCl (K+ 5mM). Wild-type (S288c) and trk1Δ trk2Δ cells (CY162) were transformed as in (a) and incubated for 5 days at 29°C.