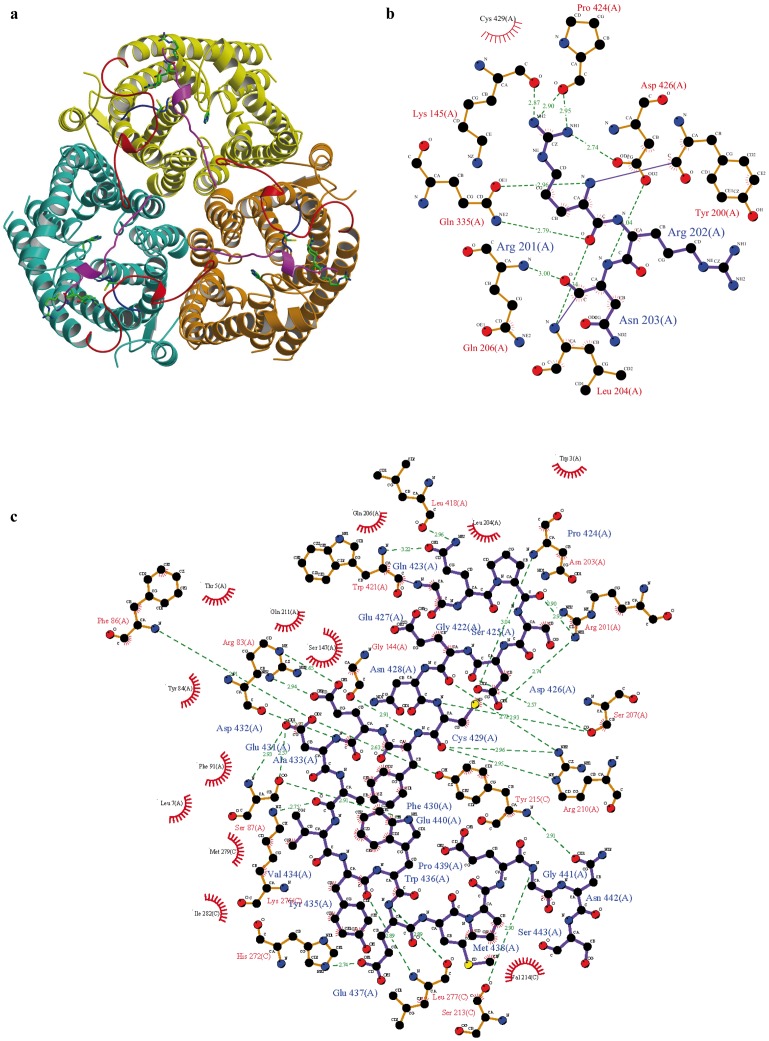
Figure 6. The C-terminal extension of HsRhCG forms a network with intracellular loops.
a A cytoplasmic view of the trimeric HsRhCG crystal structure. Each monomer is depicted in coloured ribbon. To emphasize contacts between distant residues, the long intracellular loop between transmembrane helices TM5 and TM6 is in magenta, the short loop between TM3 and TM4 in blue and the C-terminus extension from Arg417 to Ser443 is coloured in red. The C-extension of one monomer is found oriented towards the adjacent monomer, in a circular manner. Note that the last C-terminal 36 residues of HsRhCG protein cannot be built due to lack of available structural data. In order to localize the substrate pore channel, side chains of the twin-histidines (His185 and His344) are showed and labelled in each monomer. The side chain of Arg202, as well as those of the two surrounding residues, Arg201 and Asn203, are also depicted and labelled in each monomer. The atomic colour scheme is carbon in green, nitrogen in blue, oxygen in red and sulphur in yellow. b Schematic 2D interacting plot of residues Arg201 to Asn203 in HsRhCG crystal structure. Hydrogen bonds are green dashed lines and van der Waals contacts are represented by red semi-circles with radiating spokes. Bonds of Arg202 and the two surrounding residues Arg201 and Asn203 are in purple and other bonds are in light brown. All atoms and residues are labelled. The atomic colour scheme is carbon in dark, nitrogen in blue, oxygen in red and sulphur in yellow. c Schematic 2D interacting plot of residues 422 to 443 of the HsRhCG C-terminus. See (b) for the legend.