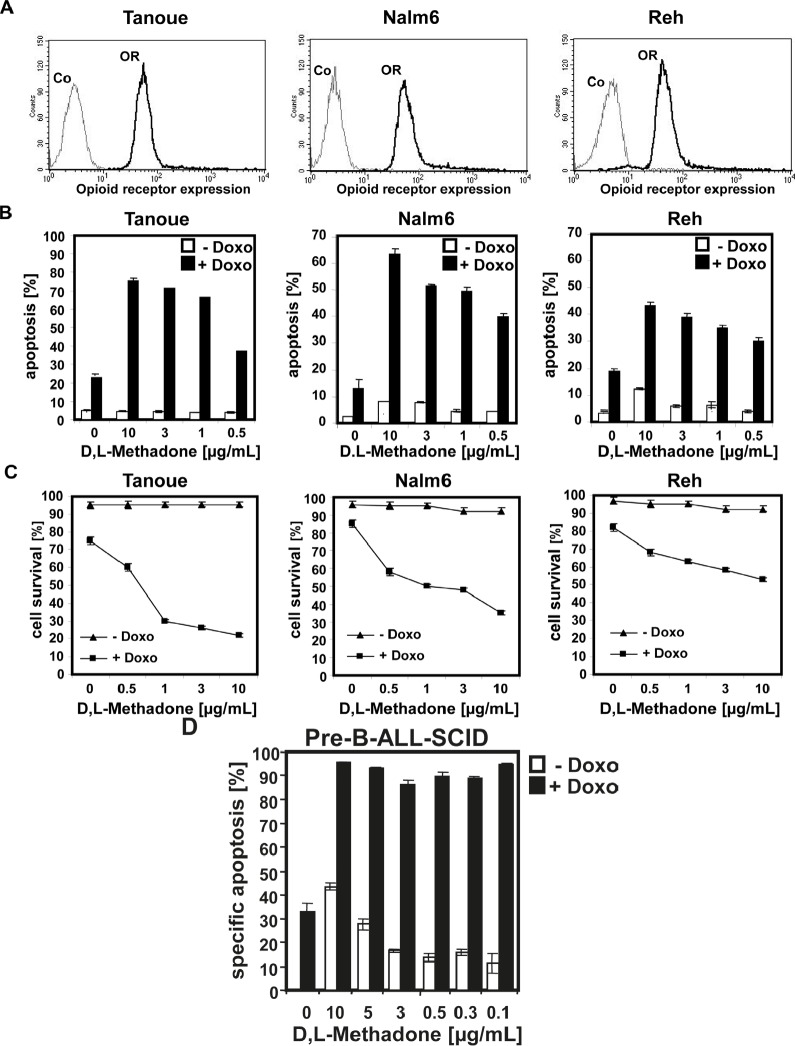
Figure 2. Combination treatment with D,L-methadone and doxorubicin induces apoptosis in ALL cells expressing moderate amounts of opioid receptors.
(a) Different BCP-ALL cell lines (Tanoue, Nalm6 and Reh) express a moderate number of opioid-receptors on their cell surface. Tanoue, Nalm6 and Reh were stained with naloxone-fluoresceine measuring opioid-receptor expression (OR, thick black curve) and analyzed by flow cytometry. Controls (Co, unstained cells) are exhibited as thin black curves. (b) BCP-ALL cell lines (Tanoue, Nalm6 and Reh) were treated with different concentrations of D,L-methadone alone (- Doxo, white columns), with doxorubicin alone or with D,L-methadone in addition to doxorubicin (+ Doxo, black columns). For the cell line Tanoue, we used doxorubicin in a concentration of 0.06μg/mL, for Nalm6 and Reh in a concentration of 0.01μg/mL. 120h after stimulation, the percentages of apoptotic cells were measured by FSC/SSC-analysis. (C) BCP-ALL cell lines (Tanoue, Nalm6 and Reh) were treated with different concentrations of D,L-methadone alone (- Doxo, triangle), with doxorubicin alone or with D,L-methadone in addition to doxorubicin (+ Doxo, square). For the cell line Tanoue, we used doxorubicin in a concentration of 0.06μg/mL, for Nalm6 and Reh in a concentration of 0.01μg/mL. 120h after stimulation, the percentages of surviving cells were measured by FSC/SSC-analysis (D). D,L-Methadone strongly enhances doxorubicin sensitivity of xenograft-derived-BCP-ALL-cells ex vivo. Xenograft-derived-BCP-ALL cells (pre-B-ALL-SCID) were treated with different concentrations of D,L-methadone (as indicated) alone (- Doxo, white columns), with 0.01μg/mL doxorubicin alone or with D,L-methadone in addition to doxorubicin (+ Doxo, black columns). 48h after stimulation, the percentages of apoptotic cells were measured by FSC/SSC-analysis. The percentage of specific apoptosis was calculated as described in Figure 1B. Columns, mean of triplicates; bars, SD<10%.