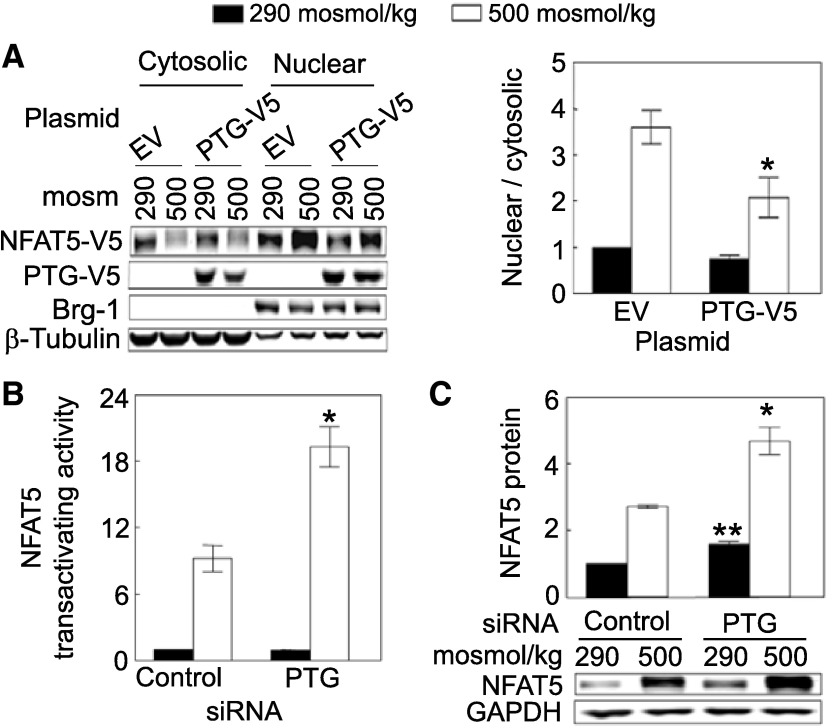
Fig. 2.
A: overexpression of PTG reduces high NaCl-induced NFAT5 nuclear localization. HEK293FT cells were transiently co-transfected with NFAT5-V5 and PTG-V5 or empty vector for 46 h, and then osmolality was increased to 500 mosM (NaCl added) or left at 290 mosM for 2 h. Proteins were extracted separately from cytoplasm and nucleus, and then analyzed by Western blotting. The cytoplasmic and nuclear proteins were consistently separated, as supported by the subcellular distributions of β-tubulin (cytoplasmic marker) and Brg-1 (nuclear marker), which are unaffected by NaCl concentration. *Signficant difference vs. control (P < 0.05; n = 4). B: siRNAs against PTG increase high NaCl-induced NFAT5 transactivating activity. As in Fig. 1B, except that we measured NFAT5 transactivating activity in HEK293 cells stably expressing a yeast binary GAL4 reporter assay system. High NaCl increases NFAT5 transactivating activity, and knockdown of PTG by its siRNAs further increases high NaCl-induced NFAT5 transactivating activity. *Signficant difference vs. control at 500 mosM (P < 0.05; n = 3). C: siRNAs against PTG increase NFAT5 protein abundance. HEK293 cells were transfected with siRNA against PTG for 32 h, and then the medium was changed for 16 h, either maintaining it at 290 mosM or increasing it to 500 mosM (NaCl added). In B and C, the PTG siRNAs decrease PTG mRNA abundance by ∼68%. *Significant difference compared with control at 500 mosM (P < 0.05; n = 3). **Significant difference compared with control at 290 mosM (P < 0.05; n = 3).