Fig. 8.
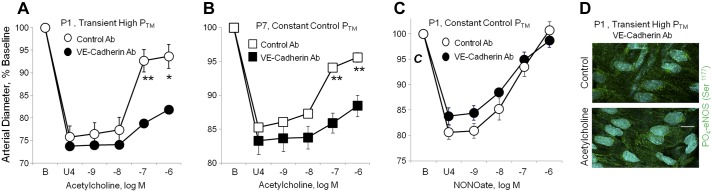
Effects of a VE-cadherin function blocking antibody on responses to acetylcholine or the NO-donor DEA-NONOate in neonatal arteries. In A, B, and C, dilator responses of paired arteries were determined in a microperfusion system (at a PTM of 20 mmHg), after they were constricted to 75–85% of baseline diameter with the thromboxane receptor agonist U46619 (U4). One artery of each pair was treated with a VE-cadherin function blocking antibody (BV13, 50 μg/ml) and the other with a control antibody (50 μg/ml). Dilator responses are expressed as a percentage of the baseline diameter (B), and presented as means + SE for n = 5 (A, C) or 4 (B). For statistical analysis, * symbols indicate a significant difference between responses in control and VE-cadherin antibody-treated arteries (1 symbol = P < 0.05; 2 symbols = P ≤ 0.01). In A, both P1 carotid arteries were exposed to a transient increase in PTM (50 mmHg, 60 min) before assessing dilator responses to acetylcholine (at 20 mmHg). The function blocking antibody to VE-cadherin markedly inhibited the response to acetylcholine. B: in P7 arteries maintained at 20 mmHg, the VE-cadherin function blocking antibody markedly inhibited the response to acetylcholine. C: in P1 arteries maintained at 20 mmHg, the function blocking antibody did not affect dilation to the NO donor DEA-NONOate. D: representative LSM fluorescent images presenting the effect of acetylcholine (10−6 M) on PO4-eNOS Ser1177 in P1 arteries, which were treated with the VE-cadherin function blocking antibody and exposed to a transient increase in PTM (50 mmHg, 60 min). PO4-eNOS Ser1177 is represented in green, while nuclei are colored light blue. Under these conditions, acetylcholine did not significantly increase levels of PO4-eNOS Ser1177 (to 105.3 + 4.9% of control, P = NS, n = 8). This contrasts with untreated P1 arteries exposed to a transient increase in PTM, where acetylcholine significantly increased PO4-eNOS Ser1177 (Fig. 6). The white line represents 10 μm.
