Figure 1.
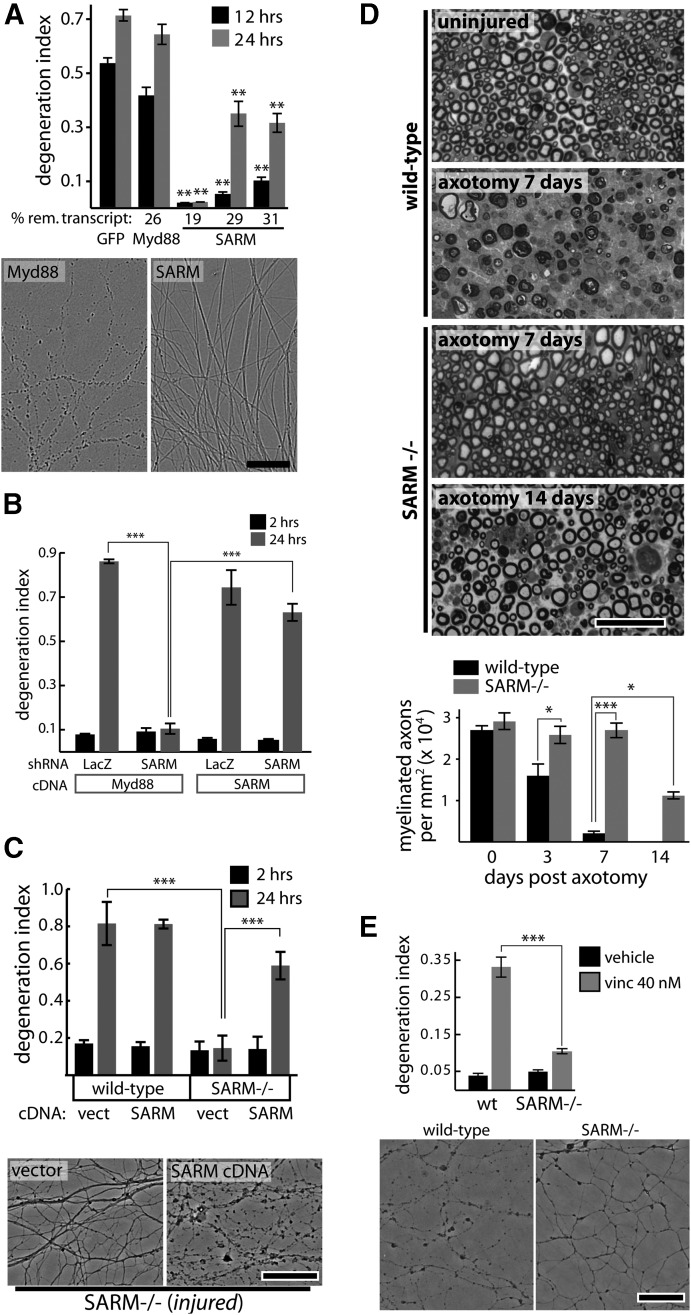
SARM is required for injury-induced axon degeneration. A, shRNAs targeting GFP (control) or Myd88 do not suppress axotomy-induced axon degeneration while SARM knockdown with three independent shRNA vectors protects axons; protection is correlated with knockdown efficiency measured by qRT-PCR (percentage remaining transcript shown below bar graph). **p < 0.01; comparisons made to shRNA sequence targeting GFP at each time point. Images of axons treated with Myd88 or SARM shRNA at 24 h postaxotomy are shown. B, Axotomy-induced axon degeneration is blocked by SARM shRNA but not control (LacZ) shRNA. Expression of human SARM cDNA does not cause direct axon degeneration (see 2 h postaxotomy) but restores normal injury-induced axon degeneration. C, Axons of wild-type DRG neurons undergo degeneration by 24 h postaxotomy while SARM−/− neurons do not. Expression of human SARM cDNA restores normal injury-induced degeneration. D, Toluidine blue-stained sciatic nerve cross sections distal to nerve transection show complete axon degeneration (loss of normal myelin profiles) by 7 d postinjury in wild-type animals while SARM−/− axons show no degeneration at 7 d and only partial degeneration by 14 d postinjury. E, Treatment with vincristine (40 nm) for 24 h causes axon fragmentation in wild-type DRG neurons but not SARM−/− neurons. Representative phase-contrast images are shown. *p < 0.05; **p < 0.01; ***p < 0.001; error bars show standard error of the mean (SEM), scale bars, 50 μm.