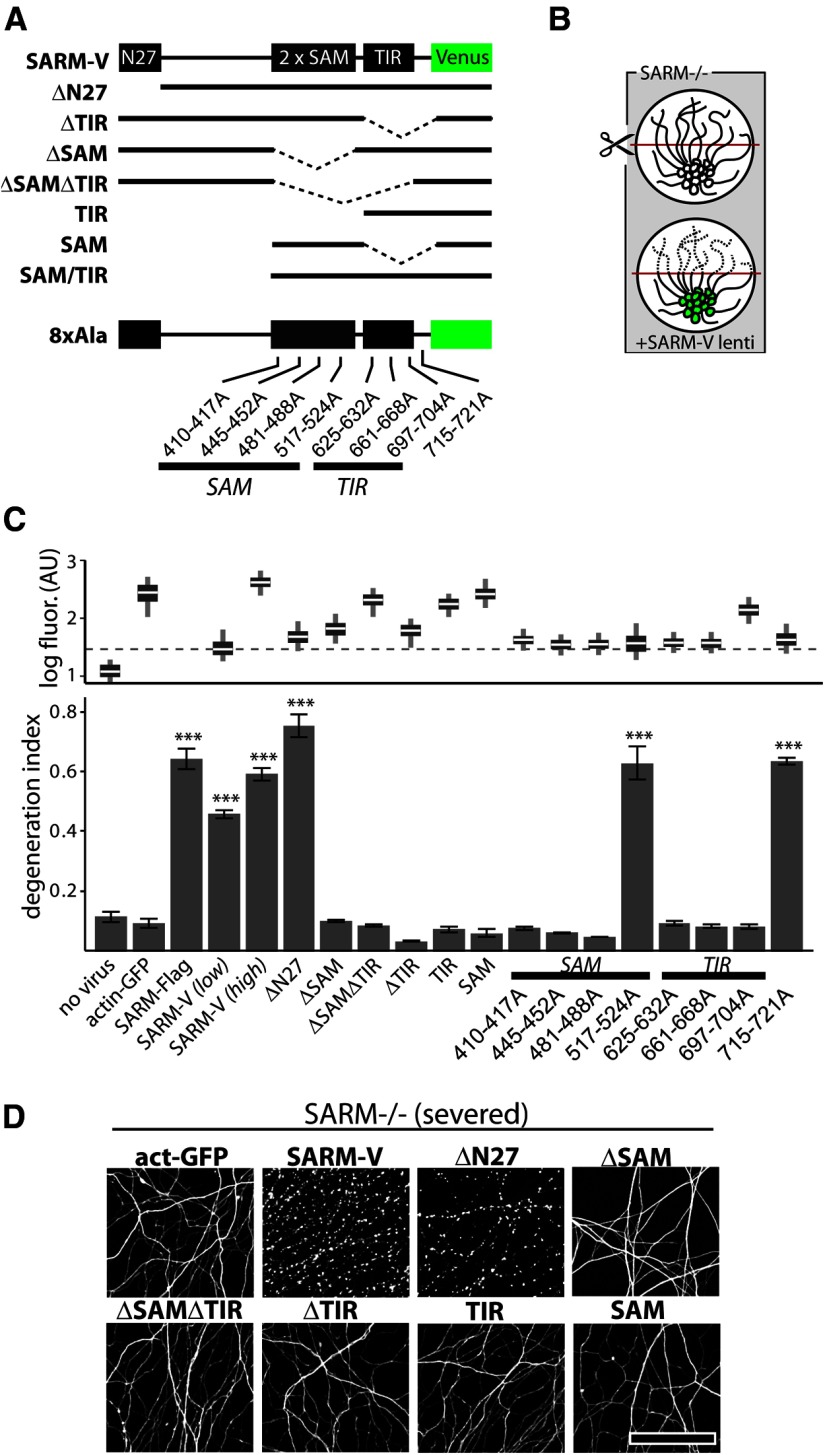
Figure 4.
SAM and TIR domains of SARM are required to mediate injury-induced axon degeneration. A, Diagram of Venus-tagged full-length SARM (SARM-V) and various mutants analyzed. Dotted lines indicate deleted regions. N27, Residues 1–27. Eight separate alanine replacement mutants (8×Ala) contain eight Ala residues in place of the indicated SARM residues. B, Genetic rescue experiment schematic: SARM−/− axons do not degenerate upon injury whereas axons of SARM−/− neurons infected with SARM lentivirus are competent to undergo injury-induced degeneration. C, Bottom, Axons of SARM−/− DRG neurons expressing actin-GFP (control) do not degenerate by 36 h postaxotomy; normal axon degeneration is restored by expression of Flag-tagged SARM (SARM-Flag), SARM-V, and ΔN27 constructs but not ΔSAM, ΔSAMΔTIR, ΔTIR, TIR, or SAM constructs. Three of four 8×Ala mutants disrupting the SAM domains and three of three disrupting the TIR domain abolished axon degeneration function of SARM while a C-terminal 8×Ala replacement outside the TIR domain did not block function. These results are also summarized in Tables 1 and 2. Top, Box plot depicts log fluorescence intensity measured from >100 cells per group (see Materials and Methods); wide boxes denote lower and upper quartiles, white lines denote median values, and whiskers extend to upper and lower 95th percentiles. Median fluorescence of all mutants exceeds that of a functional low-virus control (SARM-V low; dashed line). ***p < 0.001; one-way ANOVA with Tukey–Kramer post-test. D, Representative α-tubulin-stained axon images for deletion mutants shown in B. Error bars = SEM, scale bars, 50 μm.