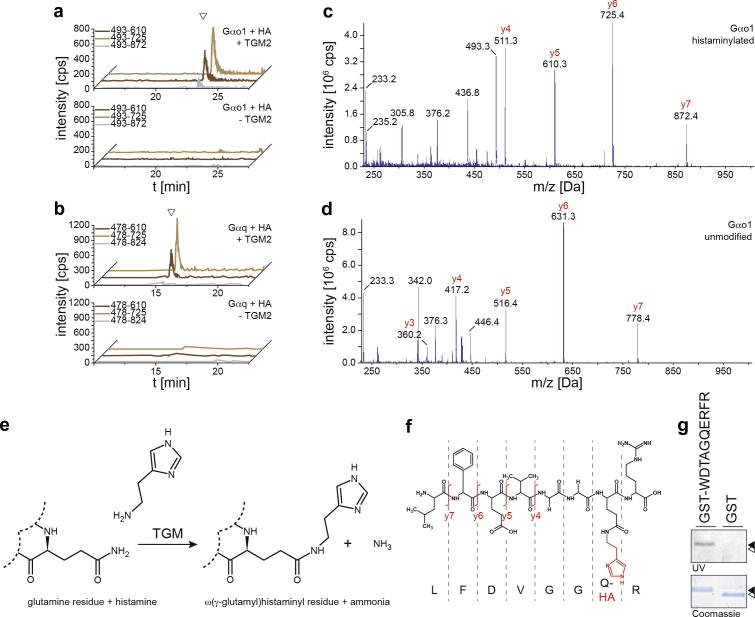
Fig. 2.
TGM2-dependent histaminylation of catalytic glutamines. (a) SRM assays identify the Gαo1 catalytic-core glutamine as recipient of histaminylation. GST-Gαo1 was incubated with 200 μM HA in the presence (upper panel) or absence (lower panel) of TGM2, digested with trypsin and analyzed by LC–MS/MS. Analyzed SRM (Q1/Q3) transitions (in m/z) are given in the legend. Please not that the corresponding Cdc42 peptide could not be analyzed as it exceeded the mass range (50–1200 Da m/z) of the mass spectrometer [39]. (b) Similar to (c), but analyzing Gαq. (c) MS/MS spectra of the histaminylated Gαo1 precursor peptide LFDVGGQHAR and (d) of the unmodified Gαo1 precursor peptide LFDVGGQR. (e) Posttranslational histaminylation of glutamine residues. Transamidation of HA to a protein-bound glutamine residue, resulting in the formation of a ω(γ-glutamyl)histaminyl residue and release of ammonia. (f) Schematic illustration of the catalytic-core Gαo1 peptide containing a sole glutamine residue, modified with histamine. (g) A linear GTPase core peptide confers TGM2 reactivity. The Rab core peptide WDTAGQERFR, conserved in several GTPases, was fused with GST. Then GST or GST-WDTAGQERFR were incubated with TGM2 and the fluorescent TGM substrate MDC. Transamidation were determined using SDS–PAGE and UV detection.