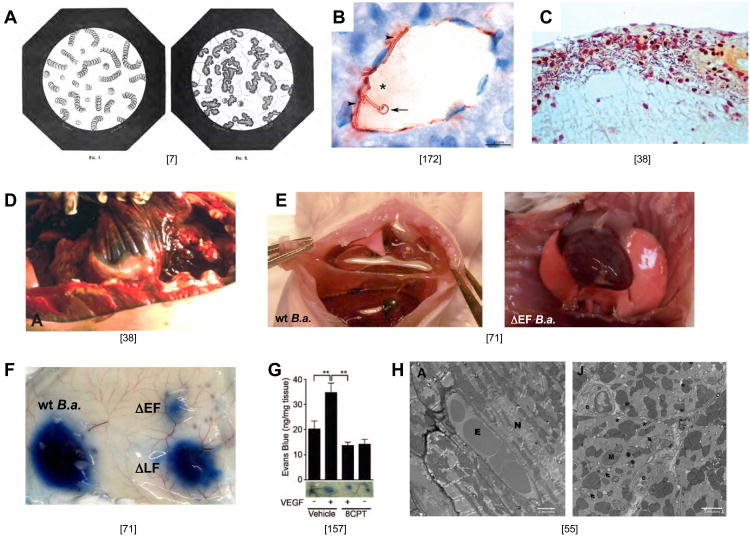
Figure 3. The fulminant phase of anthrax infection.
A) Left panel: drawing of a blood smear from an uninfected animal showing stacks of red cells (Fig. 1 from “Le charbon et la vaccination charbonneuse d'apres les traveau recents de M. Pasteur by C.H. Chamberland, 1883 [7]). Right panel (Fig. 2 from same source): Blood smear from an animal infected with B. anthracis showing aggregated masses of material and rod shaped bacteria. This phenotype could be reproduced by adding a drop of filtered cell-free liquid from blood of an anthrax infected animal to normal blood may be related to hemoconcentration or coagulopathies that have been reported in human cases of anthrax indicating the activity of a secreted substance. In Pasteur's own words: “Also, the filtered blood could be injected with impunity into the body (of a second animal) without producing anthrax or any local disturbance. However, this filtered anthrax blood, when placed in contact with fresh blood, rapidly generated agglutinated globules in as great or larger number than observed in (animals with) anthrax disease, possibly due to the presence of an enzyme formed by the bacteria.” The basis for this agglutination phenotype and whether ET or LT or neutral proteases such as InhA [184] contribute to it remains unknown. In his great summary of Pasteur's work, C.H. Chamberland also included a detailed description of the stages of anthrax disease provided by Delafond and Pasteur, which is remarkably similar to modern descriptions of the disease course in humans. These passages have been translated and condensed as follows: Delafond: “The earliest disease symptoms in a flock, which curiously would first appear in animals in their prime, were a more pronounced pink tinge of the nose and ear and small hemorrhages of blood vessels deep in the eye. During this phase lasting 2-4 days animals typically still ate and behaved normally. Blood drawn from the jugular vein as such symptoms appeared was black (a sign of anoxia), thick and agglutinated, and clotted more rapidly than normal blood (3-4 minutes versus 6-7 minutes). Next, signs of malaise were manifest: outstretched necks, dilated nostrils, difficulty breathing (dyspenea), bloody urine and moist sometimes bloody stool, and bloated stomachs after eating. The behavioral symptoms would generally subside temporarily during which time sheep would lick the rails of their enclosures or consume salt. After this short respite, disease symptoms would typically return, animals would stop eating, become lethargic, have rapid labored breath, look wild-eyed, discharge blood from their nostrils, fall to the ground, experience convulsions of all four members, and expel bloody urine and stool. Once such serious symptoms became evident, animals would expire within 5 minutes to three hours. Not all animals followed this progressive course, however. In some cases, animals that appeared to be in fine health with vigorous appetite would suddenly stop eating, lie or fall down, suffer convulsions, bleed profusely from the nose, and die in a period as brief as five minutes, seemingly by asphyxiation.” Chamberland: “In uninoculated animals similar overt signs of illness became manifest with death invariably following rapidly. In some cases, however, edema at the point of inoculation would resolve, body temperature would drop back to normal and the animals would make a full recovery, in which case the animals were almost always immune to reinfection, even with much larger doses of bacteria. The immunity of these surviving animals inspired Pasteur to conceive of vaccinating animals against anthrax with attenuated strains of bacteria.” As anthrax infected animals died, Pasteur and colleagues noted that “the cadavers had bloated abdomens, fluids leaked from all body orifices, and that they rapidly decomposed. Upon dissection of the cadavers they found lesions and hemorrhage in nearly all tissues and organs including the skin, subcutaneous tissues, lymphoid ganglia, intestinal mucosa, lungs, pancreas, kidneys, thymus, brain, choroid plexus, region surrounding the parotid and viscera cerebellum, had become swollen and gelatinous, having lost the normal morphological organization. Furthermore, all these organs while largely normal in gross structure were swollen by edema and their capillary vessels were filled with blood cells or distended with liquid. Blood leaked from larger vessels filling spaces under membranes surrounding organs such as the bronchia, intestinal mucosa, kidney, and bladder while in highly vascularized organs such as the spleen, kidney, lung, lymphoid ganglia, pancreas, thymus and choroid plexus, blood not only distended the organs engorging its vessels but escaped from the interior forming brown stains, bruises, effusion, and hemorrhaging rendering the organ a consistency of molasses that was easily torn and rent apart by pressure, oozing a dark thick blood.” B) Encapsulated B. anthracis adhere tightly to the wall of a blood vessel in the liver of an infected mouse (Fig. 3F from [172]). C) Invasion of B. anthracis into the subarachnoid space of meninges in an infected human brain (Fig. 14 from [38]). D) Gelatinous edema spreading from the mediastinum along the dorsal costal parietal pleura (Fig. 9A from [38]). E) Pleural effusion in lungs of CD-1 mice infected by IV infection with the Sterne strain of B. anthracis. Left panel WT B.a.; right panel, B.a mutant deleted for the gene encoding EF (Supp. Fig. 11C,D from [71]). F) Vascular effusion in response to subcutaneous infection with wild-type (WT) Sterne strain B. anthracis or mutants lacking the genes encoding LF (ΔLF) or EF (ΔEF) (Fig. 3p from [71]). G) Vascular effusion induced by subcutaneous injection of VEGF is reversed by activating the EPAC branch of the cAMP response revealing an in vivo barrier promoting role of cAMP. (Fig. 7D from [157]). H) Pathological changes in the hearts of LF treated mice. Left panel: EM section of heart from an untreated mouse with intact myocytes and endothelium (E = erythrocyte, N= myocyte nucleus). Right panel: Heart from an LT treated mouse (55 hours after treatment) exhibiting endothelial cell swelling, mitochondrial degeneration (arrows), and multiple swollen SR cisternae (*); M= mitochondria and e = endothelial cell. (Fig. 7A,J from [55]).