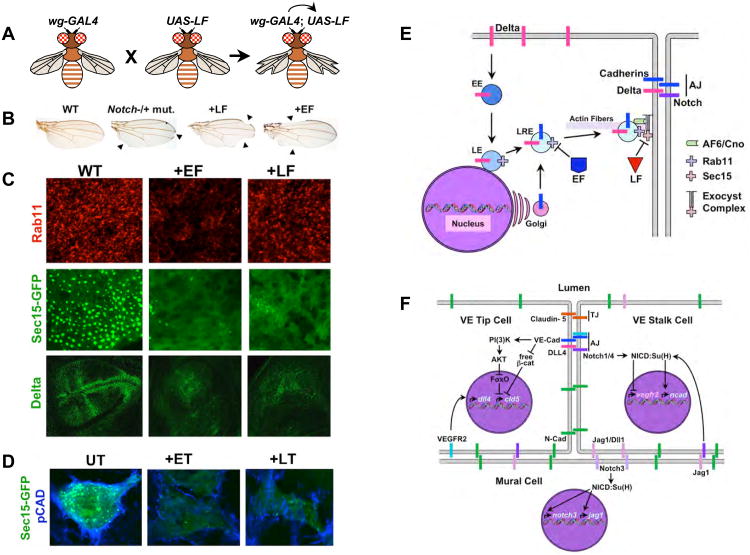
Figure 5. Anthrax toxins inhibit exocyst mediated trafficking to the AJ.
A) A schematic summary of GAL4/UAS expression system [137]. Flies (left) carrying a transgenic construct (wg-GAL4) express the yeast GAL4 transactivator protein selectively in the wings. These flies have a normal phenotype, as do flies carrying a transgenic construct in which a cDNA encoding the enzymatic portion of LF has been placed under the control of the Up-Stream-Activating (UAS) sequence (middle). When these two strains of flies are crossed to each other, their progeny (right) inherit both the wg-GAL4 construct and UAS-LF construct to which GAL4 protein can bind and activate transcription of the UAS-LF transgene in the wing, resulting in a notched wing phenotype. B) Wings from adult fruit flies: Left panel, wild-type; second panel, a heterozygous Notch mutant with 50% of wild-type activity displaying a weak notching phenotype in which sections of the edge of the wing are missing (arrowheads); third panel, fly expressing LF in wing cells has gaps in the edge of the wing (arrowheads) similar to those observed in Notch mutants; right panel, fly expressing EF in wing cells also has gaps in the edge of the wing (arrowheads). C) Fields of developing cells in the Drosophila wing primordium from wild-type flies (WT, left panels), flies expressing EF in the wing (+EF, middle panels), and flies expressing LF in the wing (+LF, right panels). Cells were stained for expression of the endogenous Rab11 or Delta proteins or for expression of a GFP-Sec15 fusion protein associated with large secretory vesicles as indicated in the figure. EF reduces the levels and activity of Rab11 resulting in loss of cell surface Sec15-GFP expression, whereas LF does not affect Rab11, but does eliminate Sec15-GFP expression at the cell surface. Thus, EF acts by blocking Rab11 function (and indirectly Sec15-GFP expression), while LF blocks Sec15-GFP expression more directly. Inhibition of exocyst activity results in greatly reduced transport of the Notch ligand Delta and Drosophila E-cadherin (not shown here, but see [71]) to the cell surface. D) ET and LT inhibit exocyst mediated trafficking in human brain microvascular endothelial cells. Pan-cadherin (blue) and Sec15-GFP (green) staining are greatly reduced by treating cells with either ET or LT. Panels A-C are assembled from figures in [71]. E) Diagram summarizing the effects of EF and LF on the exocyst. Abbreviations are as in previous figures. F) Known roles of cadherins and Notch signaling in VEC that might be disrupted as a result of anthrax toxins inhibiting exocyst mediated trafficking of these proteins to the cell surface. The first Notch-dependent interaction involved vascular remodeling in which vein inducing signals such as VEGF acting via the VEGF-R2 RTK induce expression of the DLL4 ligand in microvascular endothelial cells leading these cells to initiate formation of a new vascular outgrowth. These DLL4 expressing cells become tip cells, disengage themselves transiently from their neighbors to organize vasculogenesis, and signal to their neighbors via Notch1/Notch4 to remain as existing stalk cells. One important element of Notch signaling in stalk cells is to repress expression of VEGF-R2, since signaling via this RTK activates DLL4 expression and induces the alternative tip cell fate. In addition, VEGF-R2 activity can be inhibited post-translationally in these cells by binding to VE-Cad at the AJ, which sequesters VEGF-R2 in an inactive form. Notch signaling is also important in a reciprocal form of signaling between vascular endothelial cells and mural cells (smooth muscle cells surrounding veins, venules, arteries and arterioles, or pericytes sealing junctions between microvascular cells in impermeant capillary beds such as those constituting the blood-brain-barrier). Adhesion between VEC and mural cells depends on the type-I N-cadherin, which is not clustered in at the AJ (in distinction to VE-Cad) but rather is distributed broadly over the cell surface. N-cadherin expression in VEC depends on Notch signaling (most likely mediated by the Jagged1 expressed in mural cells activating the Notch1/Notch4 receptors in VEC). Notch signaling is also required in mural cells to promote their differentiation and is mediated by the Jagged (Jag1) or Delta-like1 (Dll1) ligands expressed in VEC signaling via the Notch3 receptor in mural cells. One effect of this signaling is to maintain the Notch signaling network by activating expression of both the Notch3 and the jagged1 genes. There is also important cross-talk between the AJ and TJ in VEC. One well documented example of this type of junctional interaction is the VE-Cad-dependent activation of Claudin-5, an essential barrier component of the VEC TJ. Clustering of VE-Cad at the AJ, which provides a strong coordinated signal, results in activation of the PI(3)K/AKT phosphorylation cascade that terminates in inactivation of the transcriptional repressor FoxO. One target gene that is otherwise repressed by FoxO in VEC is the claudin-5 (cld5) gene. VE-Cad also relieves a second form of constitutive repression of cld5 expression mediated by β-catenin, which as indicated above is accomplished by VE-Cad sequestering this potential transcriptional co-repressor at the cell surface. Blocking both FoxO and β-catenin mediated repression of cld5 is then sufficient for activation of this gene in VEC.