Figure 6.
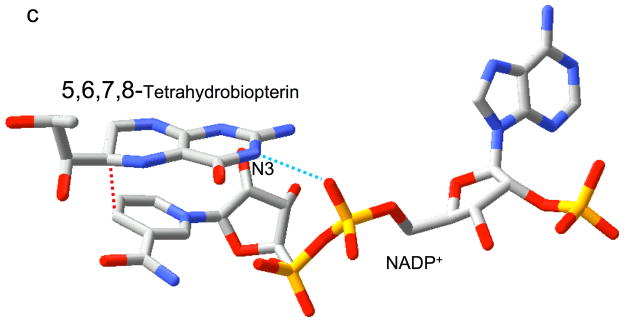
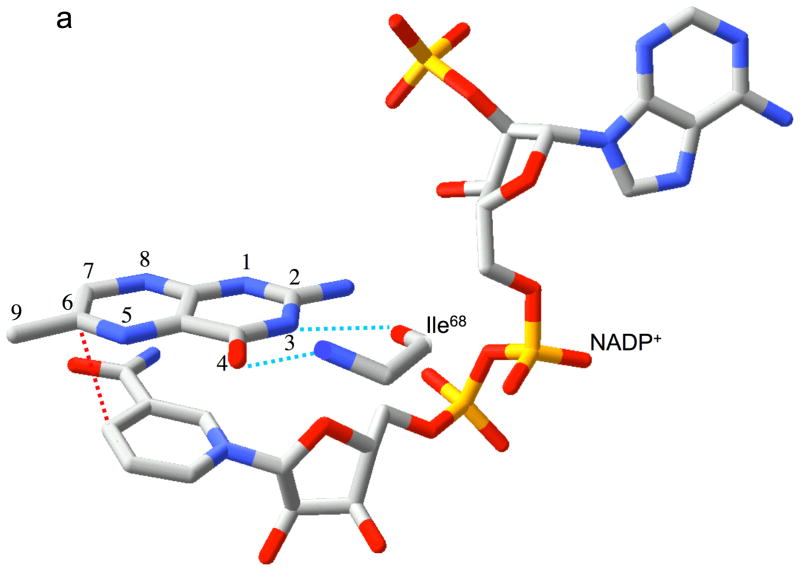
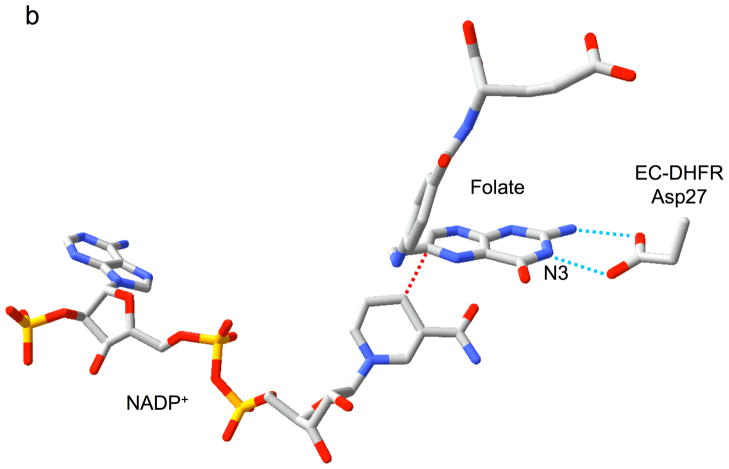
Relative substrate orientation. a) Relative orientation of NADP+ and the pteridine ring of DHF determined here for the R67 DHFR•DHF•NADP+ ternary complex. The backbone of Ile68 on chain D, which interacts with the N3-O4 amide of DHF, is also shown. The nicotinamide and pteridine ring systems adopt an endo conformation in which the closest approach corresponds to the reactive nicotinamide C4 and pteridine C6 carbons. The NADP+ is wrapped around the pteridine ring, so that the phosphates are positioned near the 2-amino group: distances: Ad-5′-P---N = 5.4 Å; Nic-5′-P---N = 6.1 Å; Ad-2′-P---N = 6.8 Å. b) Relative substrate orientation in a ternary DHFR•folate•NADP+ complex corresponding to the E. coli (Type I) enzyme (pdb entry 1RX2; (27)). The Asp-27 sidechain from the E. coli enzyme, which binds to folate N2 and N3, is also shown. The relative exo orientation contrasts with that observed for the Type II enzyme. c) Relative substrate orientation in a ternary PTR•tetrahydrobiopterin•NADP+ complex corresponding to the leishmania pteridine reductase (pdb entry 2BFP (67)). The relative endo orientation is analogous to that observed for R67 DHFR, however the enzyme catalyzes a B-side hydride transfer, so that the orientaton of the nicotinamide ring is flipped. Hydrogen bonding/salt bridge interactions with N3 are shown as blue dotted lines, and the reactive centers on the substrates are connected with red dotted lines. In order to facilitate comparison, the orientation of the pteridine ring system is similarly oriented in each frame.