Abstract
Purpose
Although the presence of cannabinoid type 1 (CB1) receptor in islets has been reported, the major contributor to the protective effect of rimonabant on islet morphology is unknown. We determined whether the protective effect of rimonabant on pancreatic islet morphology is valid in established diabetes and also whether any effect was independent of decreased food intake.
Materials and Methods
After diabetes was confirmed, Otsuka Long-Evans Tokushima Fatty rats, aged 32 weeks, were treated with rimonabant (30 mg/kg/d, rimonabant group) for 6 weeks. Metabolic profiles and islet morphology of rats treated with rimonabant were compared with those of controls without treatment (control group), a pair-fed control group, and rats treated with rosiglitazone (4 mg/kg/d, rosiglitazone group).
Results
Compared to the control group, rats treated with rimonabant exhibited reduced glycated albumin levels (p<0.001), islet fibrosis (p<0.01), and improved glucose tolerance (p<0.05), with no differences from the pair-fed control group. The retroperitoneal adipose tissue mass was lower in the rimonabant group than those of the pair-fed control and rosiglitazone groups (p<0.05). Rimonabant, pair-fed control, and rosiglitazone groups showed decreased insulin resistance and increased adiponectin, with no differences between the rimonabant and pair-fed control groups.
Conclusion
Rimonabant had a protective effect on islet morphology in vivo even in established diabetes. However, the protective effect was also reproduced by pair-feeding. Thus, the results of this study did not support the significance of islet CB1 receptors in islet protection with rimonabant in established obesity-associated type 2 diabetes.
Keywords: Cannabinoid receptor CB1, rimonabant, islet, type 2 diabetes
INTRODUCTION
Sequential hypertrophy and atrophy of pancreatic islets, accompanied with progressive disorganization and fibrosis, is characteristic of rodent obese type 2 diabetes mellitus models such as Otsuka Long-Evans Tokushima Fatty (OLETF) rats1 and Zucker Diabetes Fatty rats.2 To date, only few drugs such as thiazolinediones1,3 and rimonabant, a cannabinoid type 1 (CB1) receptor antagonist with full inverse agonist activity and high binding affinity at CB1,2,4 have been proven to preserve islet architecture in these rodent models. Recently, safety issues have been raised regarding the clinical use of thiazolinediones5-8 and there is an increasing interest in alternative drugs with protective effects on islets.
Although rimonabant was withdrawn from the market for adverse psychological effects,9 several strategies to avoid the unwanted effect are under investigation.10 Use of neutral antagonist or partial agonist of CB1 receptor, that does not block or impair constitutive CB1 activity, could maintain the metabolic benefit of rimonabant while avoiding adverse psychological effects.11,12 Another strategy is the use of peripheral CB1 receptor antagonists, which do not pass through the blood-brain barrier. Recently, it was reported that one of the peripheral CB1 receptor antagonists led to weight-independent improvements in glucose homeostasis, fatty liver, and plasma lipid profiles in a rodent pre-diabetic obesity model.13
While some of these strategies would be promising, there are some uncertain areas in the protective effect of rimonabant on islet. Firstly, previous studies have not adequately addressed whether rimonabant can protect pancreatic islets from the typical progressive disorganization and fibrosis seen in established diabetes. For example, one study focused on metabolic profiles in a pre-diabetic model without analyzing pancreatic histology,13 which other studies showed that rimonabant preserves islet architecture in rodent obese type 2 diabetes mellitus models, but the animals were not confirmed to be diabetic before the initiation of rimonabant.2,4 Secondly, previous studies did not include pair-feeding control groups in their analyses of islet morphology.2,4 Although the presence of CB1 receptors in islets14,15 and favorable direct effects of CB1 antagonism on insulin secretion in an ex vivo model16,17 have been reported, their importance in vivo has not been adequately addressed. If the protective effect of rimonabant on islet is not reproduced in pair-fed animals, it might suggest the role of islet CB1 receptor in protective effect of rimonabant on islet morphology.
The aim of this study was to reproduce the protective effect of rimonabant against morphological disintegration of islets in an animal model with established diabetes, furthermore, if the effect is reproducible, we planned to determine whether the protective effect of rimonabant is independent of reduced food intake. To this end, we analyzed the protective effect of the CB1 receptor antagonist rimonabant on islet morphology in OLETF rats which were confirmed to be diabetic before treatment. The results were compared to those in pair-fed controls to determine if a protective effect exists that is independent of reduced food intake. In addition, we also compared the results for rimonabant-treated rats to those of rats treated with rosiglitazone, an insulin-sensitizer with a known protective effect on the disintegration of islets in a rodent obese type 2 diabetes model.1,3
MATERIALS AND METHODS
Animals
Male OLETF rats and Long-Evans Tokushima Otsuka (LETO) rats, which are the lean non-diabetic counterparts to OLETF rats, were supplied at 4 weeks of age by the Otsuka Pharmaceutical Company (Tokushima, Japan). Rats were maintained at ambient temperature (22±1℃) with 12 h : 12 h light-dark cycles. We used 32-week-old male OLETF rats as an obese, overt type 2 diabetes model, since the known cumulative incidences of diabetes in male OLETF rats are 67%, 78%, and 81.2% at 4, 6, and 10 months of age, respectively.18,19 In OLETF rats (n=20), an oral glucose tolerance test (OGTT) was performed, and pretreatment glycated albumin level was measured at 32 weeks. The definition of overt diabetes was a glucose level greater than or equal to 230 mg/dL at 120 min after glucose challenge. Only rats with overt diabetes were included in this study (n=17). All animal procedures were approved by the Institutional Animal Care and Use Committee of the Kangbuk Samsung Hospital, Seoul, Republic of Korea.
Experimental design and treatment
At 32 weeks of age, diabetic OLETF and LETO rats were randomized into four groups and treated for 6 weeks: the control group (n=4 for OLETF rats, n=5 for LETO rats), rimonabant group (n=5 for OLETF rats, n=5 for LETO rats), pair-fed control group (n=4 for OLETF rats, n=5 for LETO rats), and rosiglitazone group (n=4 for OLETF rats, n=5 for LETO rats). Food intake and body weight were monitored daily during the treatment period. Rats were treated by oral gavage once a day for 6 weeks with either vehicle (PBS) for the control and pair-fed control groups, rimonabant (30 mg/kg/day, Sanofi-Aventis R&D, Paris, France) for the rimonabant group, or rosiglitazone (4 mg/kg/day, GlaxoSmithKline Pharmaceuticals, Philadelphia, PA, USA) for the rosiglitazone group. The dosage of each drug was determined based on the rat pharmacokinetic data provided by the manufacturers and previous literatures that showed metabolic efficacy with the same drugs in rat.20,21 Animals were fed standard rodent chow ad libitum except for the pair-fed control group, and all animals had free access to water throughout the experiment. The pair-fed control group did not receive rimonabant, and food intake was restricted to the same amount as the rimonabant group. After 6 weeks of treatment, we compared the results for glycated albumin, OGTT, homeostasis model assessment of insulin resistance (HOMA-IR), and adipokine levels. HOMA-IR was calculated using the following formula: HOMA-IR=[fasting serum insulin (µU/mL)]×[fasting serum glucose (mmol/L)]/22.5. Serum glycated albumin levels were measured by an enzymatic method using a Hitachi 7600 P module analyzer (Hitachi High-Technologies Corporation, Tokyo, Japan). In this method, endogenous glycated amino acid elimination reaction was followed by the glycated albumin assay using albumin-specific proteinase and ketoamine oxidase. After albumin was measured by a bromocresolpurple method, glycated albumin value was calculated as the percentage of glycated albumin in total albumin. The detailed method of this technique has been published elsewhere.22,23 Serum adiponectin levels were determined using an enzyme-linked immunosorbent assay (ELISA) kit (Adipogen Inc., Incheon, Korea), according to the manufacturer's recommendations. After sacrificing the rats by anesthetizing with carbon dioxide, we compared the masses of epididymal, mesenteric, subcutaneous, and retroperitoneal adipose tissues in each group.
OGTT
OGTTs were performed on non-anesthetized rats at 32 and 38 weeks of age. After a 16-h fast, glucose (2 g/kg as a 50% solution, w/v) was administered by oral gavage. Blood samples were obtained from snipped tails to measure blood glucose levels at 0, 15, 30, 60, 90, and 120 min after glucose administration. Glucose levels were determined using a glucometer (Glucocard X-Meter; Arkray, Kyoto, Japan). Blood samples were obtained after overnight fasting for measuring fasting serum glucose and insulin concentrations. Serum samples were immediately frozen and stored at -80℃. Glucose concentrations were measured by enzymatic assay (Sigma-Aldrich, St. Louis, MO, USA), and insulin levels were determined by an ELISA kit (Crystal Chem, Downers Grove, IL, USA).
Determination of relative beta-cell area and beta-cell proliferation
At 6 h before pancreas removal, animals were injected intraperitoneally with 100 mg/kg 5-bromo-29deoxyuridine (BrdU; Amersham, Oakville, ON, USA). Staining for insulin (DAKO, Glostrup, Denmark) and BrdU (Amersham Pharmacia Biotech, Buckinghamshire, UK) was used to assess beta-cell mass and proliferation. Paraffin-embedded pancreas tissues were sectioned serially (4 µm), and three randomly selected slides per rat tissue were used for each staining. Each section of the rat pancreas contained the whole pancreas in longitudinal dimension. After overnight incubation with rat anti-BrdU antibody (1 : 200, MCA-2060; ABd Serotec, Oxford, UK), sections were reacted with diaminobenzidine tetrahydrochloride. After overnight incubation with guinea pig anti-swine insulin antibody (1 : 500, DAKO, Glostrup, Denmark), sections were treated with a Blue Alkaline Phosphatase Substrate kit (SK-5300, Vector) and counterstained with hematoxylin. Areas with beta-cells and pancreas were determined with an aid of a ScanScope Slide Scanner (Aperio, San Diego, CA, USA) connected by video camera to a computer equipped with Image-Pro Plus software version 5.1 (Media Cybernetics, Bethesda, MD, USA). The relative cross-sectional area of beta-cells was determined by quantification of the area occupied by beta-cells and the area of all tissue in multiple fields per slide. Tissue areas were determined by marking the threshold of the captured image for brown tissue (beta-cells) and for brown and blue tissue (total tissue). In all, -70% of each section was analyzed to estimate beta-cell and total tissue area. For beta-cell proliferation, BrdU+/insulin+ cells were determined as a percentage of total insulin+ cells. Beta-cells incorporating BrdU (BrdU+) had blue/black nuclei and were counted with an aid of an Olympus BX50 microscope connected by video camera to a computer equipped with Image-Pro Plus software (Media Cybernetics, Bethesda, MD, USA).
Quantification of islet fibrosis
Fibrosis levels for each islet were quantitatively expressed on sections stained with Accustain™ Masson's Trichrome stain (HT-15, Sigma-Aldrich, St. Louis, MO, USA). The relative cross-sectional area of fibrotic change was determined by quantification of the area stained with Masson's trichrome and the area of all tissue in multiple fields per slide with a microscope, camera, and software as above. Tissue areas were determined by marking the threshold of the captured image for red tissue (fibrotic change) and for brown and blue tissue (total tissue). Beta-cell and total tissue area estimates were performed as above. The average of estimated tissue areas from two or three pancreas samples were analyzed for each rat.
Statistical analysis
Data are presented as mean±standard error of the mean. Student's t-test was used for comparisons between two groups, and one-way analysis of variance (ANOVA) with post hoc Tukey's multiple comparison test was used among multiple groups. Repeated measures ANOVA was used for analyzing the difference between repeatedly measured values among multiple groups. All statistical analyses were conducted using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA). A p-value less than 0.05 was considered statistically significant.
RESULTS
Daily food intake and body weight in LETO rats and diabetic OLETF rats
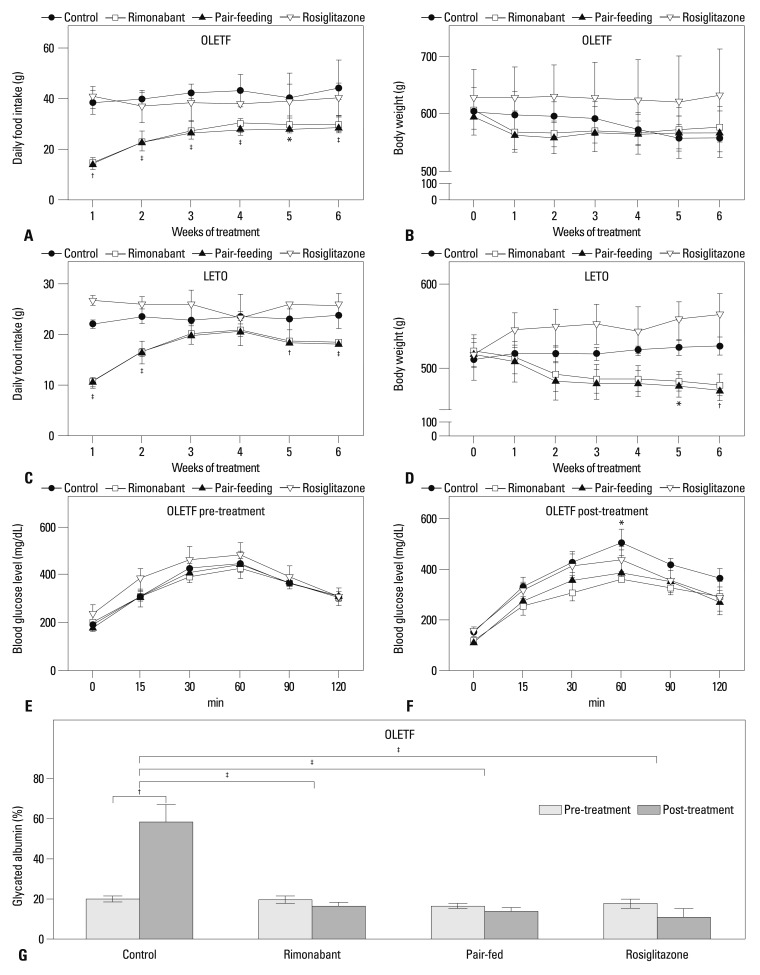
In OLETF rats, food intake of the rimonabant group was significantly less than that of both the control and rosiglitazone groups over the entire experimental period (Fig. 1A). Although there were no significant differences in body weight between groups at specific time points, body weight rapidly decreased in the first week of treatment period in the rimonabant and pair-fed control groups and no further weight loss was observed. In contrast, body weight decreased throughout the treatment period in the control group (F3,65=18.05, p>0.05 for column factor; F15,65=5.46, p<0.001 for interaction; F5,65=21.57, p<0.001 for time by repeated measures ANOVA) (Fig. 1B). In LETO rats, the rimonabant group showed decreased food intake over the treatment period except in 3rd and 4th weeks, and lower body weight at 5th and 6th weeks (Fig. 1C and D).
Fig. 1.
Food intake and weight change in OLETF (A and B) and LETO (C and D) rats over a treatment period of 6 weeks. Error bars represent standard deviation. *p<0.05 for control vs. rimonabant group; †p<0.01 for control vs. rimonabant group; ‡p<0.001 for control vs. rimonabant group. Oral glucose tolerance test before (E) and after treatment for 6 weeks (F). After treatment, the area under the curve (mg/dL×min) for each group was 48792±3340 for the control group, 36653±3692 for the rimonabant group, 39223±4156 for the pair-fed control group, and 42850±5885 for the rosiglitazone group (p=0.2591). *p<0.05 for control vs. rimonabant group. (G) Glycated albumin levels of each group before and after treatment for 6 weeks. †p<0.01; ‡p<0.001. LETO, Long-Evans Tokushima Otsuka; OLETF, Otsuka Long-Evans Tokushima Fatty.
Glucose tolerance, glycated albumin levels, relative beta cell area, and extent of islet fibrosis
There was no difference in glucose tolerance between diabetic OLETF rats in the four groups before treatment (Fig. 1E). However, after 6 weeks of treatment, glucose tolerance of the rimonabant group was better than that of the control group at 60 min (F3,65=1.46, p<0.05 for column factor, p>0.05 for both time and interaction by repeated measures ANOVA) (Fig. 1F). After 6 weeks of treatment, glycated albumin levels of the rimonabant group (16.5±2.0%), pair-fed control group (13.5±1.9%), and rosiglitazone group (11.1±8.0%) were significantly lower than those of the control group (58.3±7.7%, F3,13=21.96, p<0.001) (Fig. 1G). The glycated albumin levels of control group after the treatment was also higher than those before treatment in control group (p<0.001). No such difference was identified in the other groups. As expected, no significant difference was seen in glucose tolerance among the experimental groups in LETO rats (data not shown).
In LETO rats, the relative beta-cell area did not differ between groups and was similar to that of the rimonabant, pair-fed control, and rosiglitazone groups in OLETF rats (Fig. 2A). In OLETF rats, relative beta-cell area of control group was significantly lower than that of the counterpart control group in LETO rats (p=0.002) (Fig. 2A). In OLETF rats, the relative beta-cell area was lower in the control group than in the pair-fed control group (Fig. 2A). Insulin stain revealed intact islet morphology of LETO rats, whereas separation of endocrine cell clusters, which was most extensive in control group, was observed in OLETF rats (Fig. 2B). Therefore, further analysis of islet fibrosis and beta-cell proliferation was performed in OLETF rats. In OLETF rats, the rimonabant and pair-fed control groups showed less islet fibrosis than the control group (Fig. 3A and B). However, no difference in the extent of islet fibrosis was observed between the rimonabant and the pair-fed control groups. No groups showed differences in BrdU+/insulin+ cell number per beta-cell area (Fig. 3C).
Fig. 2.
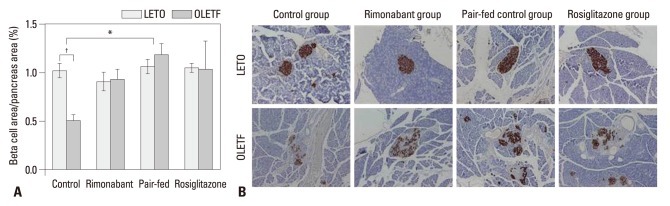
(A) Relative beta-cell area of each group after treatment. *p<0.05 for control vs. pair-fed control group in OLETF rats; †p<0.01 for control groups in LETO vs. OLETF rats. (B) Representative islet morphology after treatment. Brown indicates insulin-stained beta-cells. Original magnification, ×40. OLETF, Otsuka Long-Evans Tokushima Fatty; LETO, Long-Evans Tokushima Otsuka.
Fig. 3.
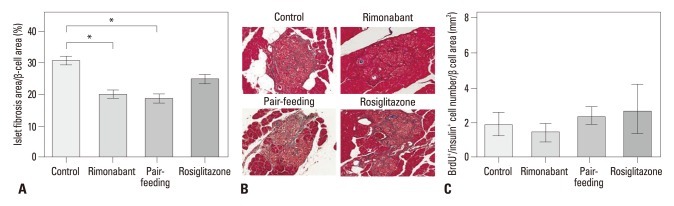
Islet fibrosis and beta-cell proliferation in OLETF rats. (A) Relative area of fibrosis in each group of OLETF rats after treatment; *p<0.01. (B) Representative islet morphology after treatment in OLETF rats. Fibrotic tissue was stained by Masson's trichrome. Original magnification ×200. (C) Average BrdU+/insulin+ cell number per beta-cell area. OLETF, Otsuka Long-Evans Tokushima Fatty.
Insulin resistance, adiponectin levels, and adipose mass
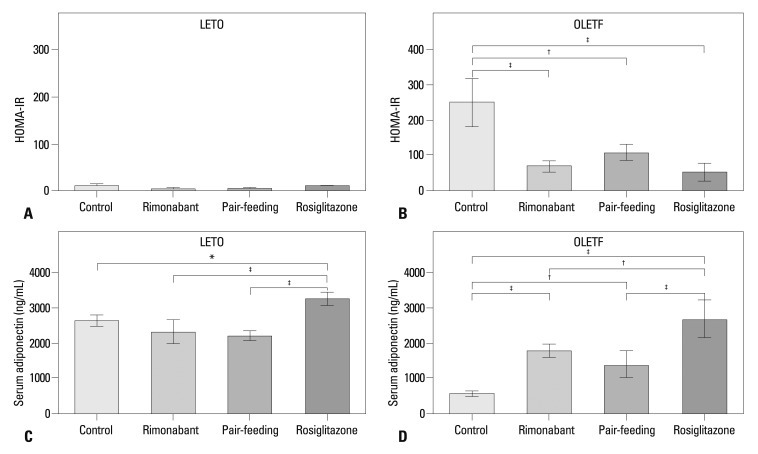
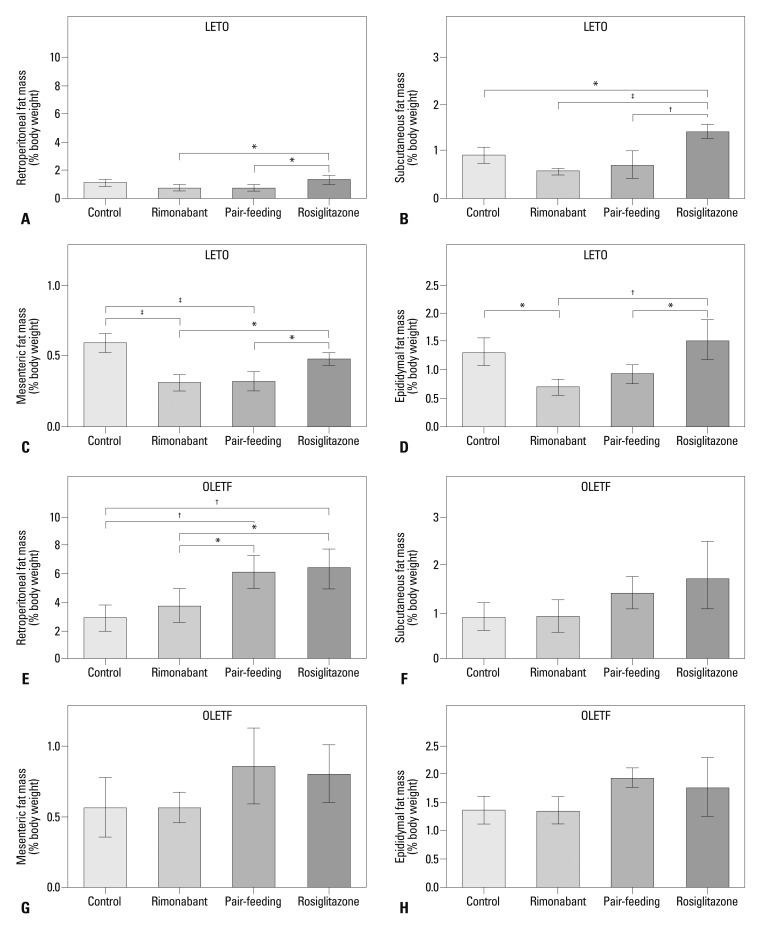
Insulin resistance in LETO rats after 6 weeks of treatment, determined by the HOMA-IR, did not differ between groups (Fig. 4A). In OLETF rats, a markedly high level of HOMA-IR was observed in control group after 6 weeks of treatment. Levels of HOMA-IR were lower in the rimonabant, pair-fed control, and rosiglitazone groups than in the control group (Fig. 4B). In both LETO and OLETF rats, serum adiponectin levels were highest in the rosiglitazone group (Fig. 4C and D). In OLETF rats, serum adiponectin levels were also higher in the rimonabant and pair-fed control groups than in the control group, with no difference between rimonabant and pair-fed control groups. In LETO rats, rimonabant and pair-feeding groups showed significantly lower retroperitoneal, subcutaneous, mesenteric, and epididymal adipose tissue mass than those of rosiglitazone group (Fig. 5A-D). Rimonabant group also showed lower mesenteric (p<0.001) (Fig. 5C) and epididymal (p<0.05) (Fig. 5D) adipose tissue mass than that of control group. In OLETF rats, the mass of retroperitoneal adipose tissue of control (p<0.01) and rimonabant groups (p<0.05) was lower than that of pair-feeding and rosiglitazone groups (Fig. 5E). The mass of retroperitoneal adipose tissue was significantly different between the rimonabant and pair-fed control groups (p<0.05) (Fig. 5E). No difference was observed in the other regions of adipose tissue in OLETF rats (Fig. 5F, G and H).
Fig. 4.
Homeostasis model assessment of insulin resistance (HOMA-IR) of each group after the treatment period in LETO (A) and OLETF (B) rats. HOMA-IR was calculated from the following formula: HOMA-IR=[fasting serum insulin (µU/mL)]×[fasting serum glucose (mmol/L)]/22.5. Fasting serum adiponectin levels of each group after the treatment period in LETO (C) and OLETF (D) rats. LETO, Long-Evans Tokushima Otsuka; OLETF, Otsuka Long-Evans Tokushima Fatty. *p<0.05; †p<0.01; ‡p<0.001.
Fig. 5.
Adipose tissue mass of each group after the treatment period. Retroperitoneal (A), subcutaneous (B), mesenteric (C), and eipididymal (D) fat mass in LETO rats. *p<0.05; †p<0.01; ‡p<0.001. Retroperitoneal (E), subcutaneous (F), mesenteric (G), and eipididymal (H) fat mass in OLETF rats. *p<0.05; †p<0.01. OLETF, Otsuka Long-Evans Tokushima Fatty; LETO, Long-Evans Tokushima Otsuka.
DISCUSSION
In this study, rimonabant treatment reduced food intake, glycated albumin levels, and insulin resistance, and transiently improved glucose tolerance in diabetic OLETF rats. Rimonabant also reduced islet fibrosis in vivo even after the establishment of diabetes, as shown in pre-diabetic obesity models of previous studies. However, this protective effect was not different from that in the pair-fed control group.
In both LETO and diabetic OLETF rats, rimonabant significantly reduced food intake. Although difference in body weight was not significant in diabetic OLETF rats because the control lost body weight due to severe hyperglycemia (Fig. 1G), reduced food intake by rimonabant and pair-feeding was sufficient to cause significant metabolic benefit such as prevention of post-treatment hyperglycemia, protection from islet fibrosis, and lower levels of insulin resistance in diabetic OLETF rats. Interestingly, treatment with rimonabant transiently improved glucose tolerance despite a lack of significant differences in relative beta-cell area. This could at least in part be explained by the improved insulin sensitivity in rimonabant group. Another possibility is that antagonism of the CB1 receptor might potentiate insulin secretion and attenuate glucagon secretion. Although a previous static incubation study showed conflicting results, a perifusion study showed that CB1 antagonism can stimulate insulin secretion.24 In isolated human islets, CB1 receptor antagonism blocked CB1-induced stimulation of glucagon and somatostatin secretion.25
In this study, rimonabant protected OLETF rats against morphological degradation and fibrosis of islets even when treatment was initiated after establishment of diabetes. Because there was no difference between rimonabant and pair-feeding groups, the mechanism of reduced islet fibrosis involves likely improved systemic insulin sensitivity, rather than direct action via islet CB1 receptor. Histologic changes in the pancreas of OLETF rats have been suggested to be the result of over-activity of beta-cells to compensate for insulin resistance.19 The reduced levels of HOMA-IR in rimonabant and pair-feeding groups in diabetic OLETF rats, which were comparable to that of rosiglitazone group, could explain the reduced islet fibrosis. However, there were several different features in adiponection levels and adipose tissue mass between rimonabant/pair-feeding groups and rosiglitazone group. While rosiglitazone group was characterized by increased adiponectin levels in both LETO and OLETF rats, rimonabant and pair-feeding groups showed lower levels of adiponectin than that of rosiglitazone group in both LETO and OLETF rats. In contrast, rimonabant group was characterized by reduced epididymal and mesenteric fat mass in LETO rats, and reduced retroperitoneal fat mass in diabetic OLETF rats. Interestingly, the retroperitoneal fat mass of rimonabant group in diabetic OLETF rats was significantly lower than that of pair-feeding group. These results are consistent with previous studies showing that rimonabant significantly enhances lipolysis in diet-induced obesity, directly leading to a reduction in adipose tissue mass.26,27 Collectively, the action of rimonabant on adipose tissue, which was different from that of rosiglitazone, renews the clinical interest in CB1 pathway as a potential target of new insulin-sensitizing agent in diabetic patients. In addition, although the presence of CB1 receptors in islets24,25 and the favorable direct effects of CB1 antagonism on insulin secretion in an ex vivo model17 have been reported, the lack of differences in islet fibrosis between the rimonabant and pair-fed control groups in this study did not support significance of their role in islet protection with rimonabant.
Several limitations of this study should be addressed. Firstly, the sample size would be insufficient to demonstrate subtle difference between groups, although the results of this study showed some positive findings in several outcomes. Secondly, diabetic OLETF rats in this study do not represent general population with type 2 diabetes. In OLETF rats, satiety deficit, which might come from the lack of cholecystokinin (CCK)-A receptors, leads to increases in meal size, overall hyperphagia, and obesity.28 Therefore, the metabolic benefit of rimonabant in this study is more relevant to the type 2 diabetes patients with severe obesity, in whom correction of hyperphagia by education is difficult. Thirdly, the metabolic benefit of rimo-nabant in diabetic OLETF groups should be interpreted in the context that glucotoxicity was present in the control group in OLETF rats.
In conclusion, rimonabant had a protective effect on pancreas islet morphology in vivo, even when the treatment was initiated after the establishment of diabetes. The main contributor of this protective effect was reduced food intake, which resulted in improved insulin sensitivity and less glucotoxicity. The present results did not indicate the significance of islet CB1 receptors in the prevention of morphological degradation of islets by rimonabant in obesity-associated type 2 diabetes.
ACKNOWLEDGEMENTS
This work was supported by grants from the Samsung Biomedical Research Institute (Project SBRI C-B0-309-1), the Korean Diabetes Association (2007), and the Ministry of Health & Welfare, Republic of Korea (Project A110741). The funding agencies had no roles in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Parts of this study were presented in abstract form at the 3rd Scientific Meeting of the Asian Association for the Study of Diabetes, Beijing, China, July 22-24, 2011.
The authors have no financial conflicts of interest.
References
- 1.Koh EH, Kim MS, Park JY, Kim HS, Youn JY, Park HS, et al. Peroxisome proliferator-activated receptor (PPAR)-alpha activation prevents diabetes in OLETF rats: comparison with PPAR-gamma activation. Diabetes. 2003;52:2331–2337. doi: 10.2337/diabetes.52.9.2331. [DOI] [PubMed] [Google Scholar]
- 2.Duvivier VF, Delafoy-Plasse L, Delion V, Lechevalier P, Le Bail JC, Guillot E, et al. Beneficial effect of a chronic treatment with rimonabant on pancreatic function and beta-cell morphology in Zucker Fatty rats. Eur J Pharmacol. 2009;616:314–320. doi: 10.1016/j.ejphar.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 3.Finegood DT, McArthur MD, Kojwang D, Thomas MJ, Topp BG, Leonard T, et al. Beta-cell mass dynamics in Zucker diabetic fatty rats. Rosiglitazone prevents the rise in net cell death. Diabetes. 2001;50:1021–1029. doi: 10.2337/diabetes.50.5.1021. [DOI] [PubMed] [Google Scholar]
- 4.Janiak P, Poirier B, Bidouard JP, Cadrouvele C, Pierre F, Gouraud L, et al. Blockade of cannabinoid CB1 receptors improves renal function, metabolic profile, and increased survival of obese Zucker rats. Kidney Int. 2007;72:1345–1357. doi: 10.1038/sj.ki.5002540. [DOI] [PubMed] [Google Scholar]
- 5.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 6.Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA. 2007;298:1189–1195. doi: 10.1001/jama.298.10.1189. [DOI] [PubMed] [Google Scholar]
- 7.Lewis JD, Ferrara A, Peng T, Hedderson M, Bilker WB, Quesenberry CP, Jr, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011;34:916–922. doi: 10.2337/dc10-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piccinni C, Motola D, Marchesini G, Poluzzi E. Assessing the association of pioglitazone use and bladder cancer through drug adverse event reporting. Diabetes Care. 2011;34:1369–1371. doi: 10.2337/dc10-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007;370:1706–1713. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- 10.Bermudez-Silva FJ, Viveros MP, McPartland JM, Rodriguez de Fonseca F. The endocannabinoid system, eating behavior and energy homeostasis: the end or a new beginning? Pharmacol Biochem Behav. 2010;95:375–382. doi: 10.1016/j.pbb.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Pavon FJ, Bilbao A, Hernández-Folgado L, Cippitelli A, Jagerovic N, Abellán G, et al. Antiobesity effects of the novel in vivo neutral cannabinoid receptor antagonist 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-3-hexyl-1H-1,2,4-triazole--LH 21. Neuropharmacology. 2006;51:358–366. doi: 10.1016/j.neuropharm.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 12.Salamone JD, McLaughlin PJ, Sink K, Makriyannis A, Parker LA. Cannabinoid CB1 receptor inverse agonists and neutral antagonists: effects on food intake, food-reinforced behavior and food aversions. Physiol Behav. 2007;91:383–388. doi: 10.1016/j.physbeh.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tam J, Vemuri VK, Liu J, Bátkai S, Mukhopadhyay B, Godlewski G, et al. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest. 2010;120:2953–2966. doi: 10.1172/JCI42551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakata M, Yada T. Cannabinoids inhibit insulin secretion and cytosolic Ca2+ oscillation in islet beta-cells via CB1 receptors. Regul Pept. 2008;145:49–53. doi: 10.1016/j.regpep.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Vilches-Flores A, Delgado-Buenrostro NL, Navarrete-Vázquez G, Villalobos-Molina R. CB1 cannabinoid receptor expression is regulated by glucose and feeding in rat pancreatic islets. Regul Pept. 2010;163:81–87. doi: 10.1016/j.regpep.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 16.Getty-Kaushik L, Richard AM, Deeney JT, Krawczyk S, Shirihai O, Corkey BE. The CB1 antagonist rimonabant decreases insulin hypersecretion in rat pancreatic islets. Obesity (Silver Spring) 2009;17:1856–1860. doi: 10.1038/oby.2009.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li C, Bowe JE, Huang GC, Amiel SA, Jones PM, Persaud SJ. Cannabinoid receptor agonists and antagonists stimulate insulin secretion from isolated human islets of Langerhans. Diabetes Obes Metab. 2011;13:903–910. doi: 10.1111/j.1463-1326.2011.01422.x. [DOI] [PubMed] [Google Scholar]
- 18.Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992;41:1422–1428. doi: 10.2337/diab.41.11.1422. [DOI] [PubMed] [Google Scholar]
- 19.Ishida K, Mizuno A, Min Z, Sano T, Shima K. Which is the primary etiologic event in Otsuka Long-Evans Tokushima Fatty rats, a model of spontaneous non-insulin-dependent diabetes mellitus, insulin resistance, or impaired insulin secretion? Metabolism. 1995;44:940–945. doi: 10.1016/0026-0495(95)90249-x. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe T, Kubota N, Ohsugi M, Kubota T, Takamoto I, Iwabu M, et al. Rimonabant ameliorates insulin resistance via both adiponectin-dependent and adiponectin-independent pathways. J Biol Chem. 2009;284:1803–1812. doi: 10.1074/jbc.M807120200. [DOI] [PubMed] [Google Scholar]
- 21.Yang SJ, Choi JM, Chae SW, Kim WJ, Park SE, Rhee EJ, et al. Activation of peroxisome proliferator-activated receptor gamma by rosiglitazone increases sirt6 expression and ameliorates hepatic steatosis in rats. PLoS One. 2011;6:e17057. doi: 10.1371/journal.pone.0017057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kouzuma T, Usami T, Yamakoshi M, Takahashi M, Imamura S. An enzymatic method for the measurement of glycated albumin in biological samples. Clin Chim Acta. 2002;324:61–71. doi: 10.1016/s0009-8981(02)00207-3. [DOI] [PubMed] [Google Scholar]
- 23.Kouzuma T, Uemastu Y, Usami T, Imamura S. Study of glycated amino acid elimination reaction for an improved enzymatic glycated albumin measurement method. Clin Chim Acta. 2004;346:135–143. doi: 10.1016/j.cccn.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 24.Li C, Bowe JE, Jones PM, Persaud SJ. Expression and function of cannabinoid receptors in mouse islets. Islets. 2010;2:293–302. doi: 10.4161/isl.2.5.12729. [DOI] [PubMed] [Google Scholar]
- 25.Bermúdez-Silva FJ, Suárez J, Baixeras E, Cobo N, Bautista D, Cuesta-Muñoz AL, et al. Presence of functional cannabinoid receptors in human endocrine pancreas. Diabetologia. 2008;51:476–487. doi: 10.1007/s00125-007-0890-y. [DOI] [PubMed] [Google Scholar]
- 26.Vettor R, Pagano C. The role of the endocannabinoid system in lipogenesis and fatty acid metabolism. Best Pract Res Clin Endocrinol Metab. 2009;23:51–63. doi: 10.1016/j.beem.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Mølhøj S, Hansen HS, Schweiger M, Zimmermann R, Johansen T, Malmlöf K. Effect of the cannabinoid receptor-1 antagonist rimonabant on lipolysis in rats. Eur J Pharmacol. 2010;646:38–45. doi: 10.1016/j.ejphar.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Moran TH, Katz LF, Plata-Salaman CR, Schwartz GJ. Disordered food intake and obesity in rats lacking cholecystokinin A receptors. Am J Physiol. 1998;274(3 Pt 2):R618–R625. doi: 10.1152/ajpregu.1998.274.3.R618. [DOI] [PubMed] [Google Scholar]