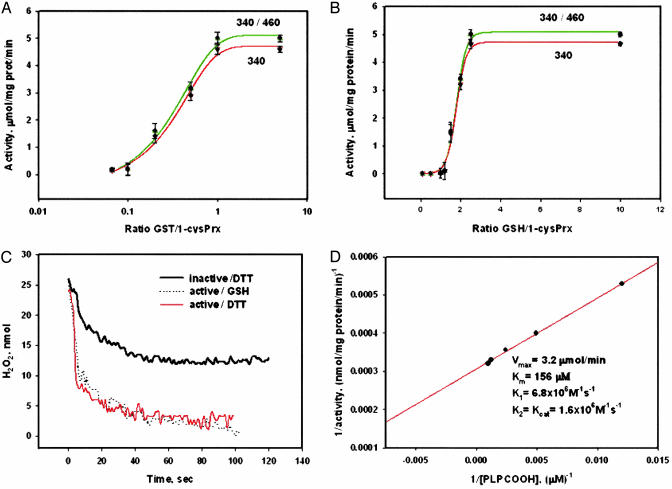
Fig. 3.
Biochemical characterization of 1-cysPrx activation. (A) enzymatic activity of recombinant 1-cysPrx as a function of GST/1-cysPrx as detected by NADPH fluorescence (340/460, green) or absorbance (340, red) with PLPCOOH as substrate (mean ± SE, n = 3). (B) Same as A but varying GSH/1-cysPrx with GST/1-cysPrx = 1. (C) Enzymatic activity of 1-cysPrx with H2O2 substrate detected by real-time absorbance (340 nm). Activity of 1-cysPrx in the presence of 88 μM DTT (black trace), and GST-activated enzyme with either 100 μM GSH (dot trace) or with 88 μM DTT (red trace). (D) Double reciprocal plot and kinetic constants of GST-activated 1-cysPrx with PLPCOOH as substrate.