Table 2.
Timeliness and accuracy of electronic laboratory reporting by reportable disease: North Carolina, January–March 2012
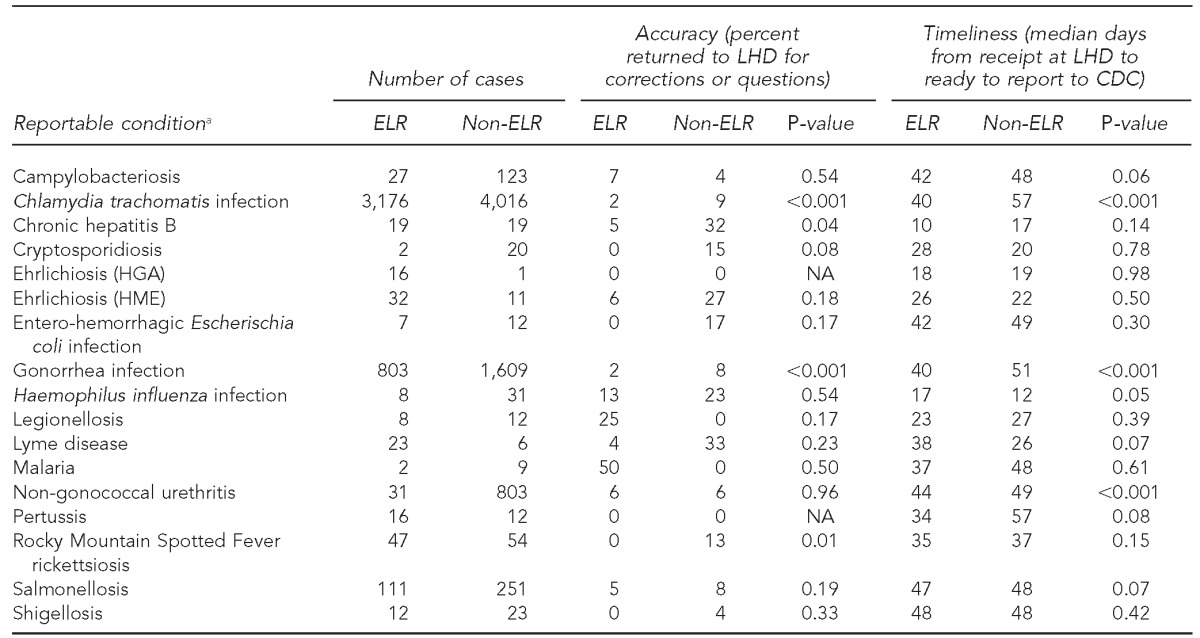
aConditions for which cases were present in the NC EDSS database but not reported by ELR were arboviral encephalitis (non-West Nile or La Crosse), brucellosis, Clostridium perfringens infections, Creutzfeldt-Jakob disease, dengue fever, diphtheria, hepatitis A, perinatal hepatitis B, acute hepatitis, influenza, La Crosse (California) encephalitis, Hansen disease (Leprosy), leptospirosis, measles, pelvic inflammatory disease, pneumococcal meningitis, Q fever, rubella, Staphylococcus aureus with reduced susceptibility to vancomycin, invasive group A streptococcal infection, streptococcal toxic shock syndrome, acute typhoid fever, vaccinia, and West Nile virus encephalitis. Some cases, such as hepatitis A, are submitted by ELR but are also submitted by fax and are prioritized for rapid hand entry when the fax arrives (generally, sooner than when the ELR record is received). Therefore, ELR is not the initial method of receipt for these cases.
LHD = local health department
CDC = Centers for Disease Control and Prevention
ELR = electronic laboratory reporting
HGA = human granulocytic anaplasmosis
NA = not applicable
HME = human monocytic ehrlichiosis
NC EDSS = North Carolina Electronic Disease Surveillance System
