Abstract
Purpose.
To develop instrumentation and methods for thorough quantitative assessment of the pupillary light reflex (PLR) in dogs under varying stimulus conditions.
Methods.
The PLR was recorded in normal Dachshunds using a custom system allowing full user control over stimulus intensity, color, and duration. Chemical restraint protocols were compared to determine which protocol provided for optimal baseline stability of pupil size and appropriate eye positioning. A series of white light stimuli of increasing intensity was used to elicit pupil constriction. Pupil images were concurrently recorded using continuous infrared illumination and an infrared-sensitive camera. The PLR was also recorded in response to blue and red stimuli.
Results.
With injectable chemical restraint alone, spontaneous fluctuations in pupil size occurred independent of light stimulation, and spontaneous eye movements made it difficult to fully visualize the pupil. Combined injectable chemical and inhalation restraint provided a steady baseline pupil size throughout PLR assessment and allowed for stable positioning of the eye using a conjunctival stay suture. Robust PLRs were elicited with all light colors. PLR constriction amplitude increased with increasing flash intensity and ranged from 5% to 70%.
Conclusions.
A recording system and protocol have been developed to reliably quantify the canine PLR. The techniques and instrumentation will be useful for objective quantitative assessment of the PLR in dogs and other species in research applications and may be useful in clinical veterinary ophthalmology and neurology if PLR abnormalities detected with these procedures can be associated with specific diseases.
Keywords: animal model, dog, electroretinogram, melanopsin, pupillary light reflex
Methods and instrumentation have been developed to quantitatively assess the canine pupillary light reflex (PLR). Recordable PLRs could be elicited with 10% of the light required to elicit detectable electroretinogram responses.
Introduction
Assessment of the pupillary light reflex (PLR) has long been used to assist in disease diagnosis in both human and veterinary medicine.1,2 However, routine clinical examinations usually employ only gross observations of the pupillary responses to light that are largely qualitative. Such PLR assessments can be used only as discriminating measures when substantial deficits in retinal or autonomic function are present. Recently, pupillography, or quantitative analysis of the PLR, has been developed as an objective technique capable of detecting subtle changes associated with the complex network of neuronal circuitry involved in modulating pupil size.3,4 Changes in PLR parameters, such as constriction amplitude and latency, have been associated with retinal dysfunction, including degeneration of the rod and cone photoreceptors,3,5,6 inner retina,7,8 and ganglion cells.9 However, pupillography is not limited to detecting retinal deficits but has also proved an indicator of autonomic dysfunction.10–15
Canine homologues have the potential to serve as important models of human hereditary retinal and neurological diseases. Canine and human retinas are very similar with respect to both anatomy and physiology. There are only two substantial differences between the canine and human retinas. Human retinas have three cone subtypes with opsins of different spectral sensitivities, whereas dogs have only two classes of cones sensitive to long/medium wavelength light (555 nm peak sensitivity; red/green or L/M-opsin) and short wavelength light (429 nm peak sensitivity; blue or S-opsin).16,17 Although canine and human retinas both have a specialized area of high visual acuity, the canine area centralis does not contain a region consisting exclusively of cones that is comparable to the human fovea.18 Although this may limit the dog as a model of macular dystrophies, for most aspects of retinal structure and function, including the PLR, these differences are not significant and data obtained from dogs would likely be applicable to humans. The neural pathways involved in mediating the PLR are also quite similar in dogs and humans. Thus, study of the PLR in canine models can lead to better understanding of the mechanisms underlying disease and can facilitate evaluation of the efficacy of potential treatments for retinal and neurological diseases in both dogs and people.
Electroretinography (ERG) is commonly used to assess retinal function in both dogs and people, but the ERG evaluates only the initial portions of the pathways involved in retinal-mediated responses to light stimuli. The PLR can be used to complement ERG assessments for objective quantitative evaluation of the integrity of neural pathways originating in the retina. To make optimal use of dogs as models for diseases affecting function of the retina and associated central nervous system pathways, objective measures to assess the functional integrity of these pathways would be of great benefit. Although the PLR has been widely used in people, pupillography has not been extensively studied in dogs or other large animals due to several obstacles. Physical restraint of the animal during recording causes stress or anxiety, which can influence the PLR.19 In addition, with a conscious, unsedated animal, it is difficult to keep the globe of the eye stationary, as is required for pupillography. Unlike people, animals will not voluntarily maintain visual fixation on a target without extensive training. Such training is not practical in most clinical or research settings.
To overcome these barriers and facilitate the use of pupillography in evaluation of potential canine models of visual system and other neurological disorders, studies were undertaken to develop a reliable protocol for quantitative assessment of the PLR in dogs under varying stimulus conditions.
Methods
Animals
Studies employed normal purpose–bred long-haired miniature Dachshunds (n = 15) housed in a research facility at the University of Missouri. Before inclusion in the study, all dogs received a complete ophthalmic examination, including vision assessment, slit lamp biomicroscopy, indirect ophthalmoscopy, and retinal photography. Dogs with evidence of vision compromise or ophthalmic conditions deemed threatening to vision were excluded. Dogs were entrained to a 12:12 daily light cycle and were socialized daily in addition to receiving routine husbandry care. All studies were performed in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the University of Missouri Animal Care and Use Committee.
Experimental Design
Dogs underwent PLR recordings using either injectable chemical restraint alone (n = 8), or chemical restraint using a combination of injectable and inhalant compounds (n = 7). For the group that received injectable chemical restraint alone, each method described below was tested on at least two separate occasions in different dogs. No single dog was subjected to recordings more often than once per week. For dogs that received the combination of injectable and inhalant chemical restraint, a standard PLR recording session was repeated in each dog every 2 months from 4 to 12 months of age, for a total of five recordings per dog.
Chemical Restraint for PLR Recording
An initial goal of these studies was to record the PLR under chemical restraint that is as similar as possible to the type of injectable restraint widely used to record the ERG.20–24 In attempts to achieve this goal, five methods of chemical restraint were assessed for use in quantitative evaluation of the PLR. Four methods involved injectable chemical restraint (CR) only, and the fifth method used injectable CR in combination with inhalant CR. For all methods, 30 minutes of dim light adaptation preceded drug administration (0.9 lux broad-spectrum, diffuse white light from a Burton model 0134500 fixture equipped with a 150-Watt halogen bulb, color temperature 3150°K [Burton Industries, Hazelhurst, WI]). The methods employing only injectable chemical restraint are widely used in veterinary medicine and are as follows: low-dose dexmedetomidine (5 μg/kg intramuscularly [IM]; Dexdomitor; Orion Corp., Espoo, Finland) (method CR1), high-dose dexmedetomidine (35 μg/kg IM) (method CR2), combined dexmedetomidine (18 μg/kg IM) and ketamine (3.5 mg/kg IM; KetaVed; Vedco, Inc., Saint Joseph, MO) (method CR3), or combined dexmedetomidine (5 μg/kg IM) and butorphanol (0.17 mg/kg IM; Torbugesic; Fort Dodge Animal Health, Overland Park, KS) (Method CR4).
For the combined injectable and inhalant chemical restraint (method CR5), dexmedetomidine (20–25 μg/kg IM) was given 30 minutes before induction of anesthesia with propofol (intravenous [IV] to effect, 1.49 ± 0.59 mg/kg [mean ± SD]; PropoFlo 28; Abbott Laboratories, Abbott Park, IL). Dogs were intubated with a cuffed endotracheal tube and restraint maintained with isoflurane (1.5% vaporizer setting; Terrell; Piramal Healthcare, Boise, ID) in oxygen. Vaporizer calibration was verified annually by an authorized anesthesia service technician.
Heart and respiratory rates were monitored throughout all procedures, and body temperature was maintained between 98.0°F and 101.5°F using a heated circulating water blanket. In addition, while dogs were under CR5, IV fluids were administered (5 mL/kg/h; veterinary lactated ringer's injection USP), and a portable multiparameter veterinary monitor (Cardell 9500 HD; Midmark Corp., Versailles, OH) was used to assess blood pressure, oxygen saturation, and end-tidal CO2 levels throughout the procedure.
The methods of chemical restraint were evaluated with regard to whether the level of restraint was adequate to perform the required procedures without causing stress to the dog. Signs of stress in the dog included a sudden increase in respiration or heart rate and physical resistance to the placement of a speculum or stay suture. Stability of the baseline pupil size was also evaluated.
PLR Recording Apparatus and Protocol
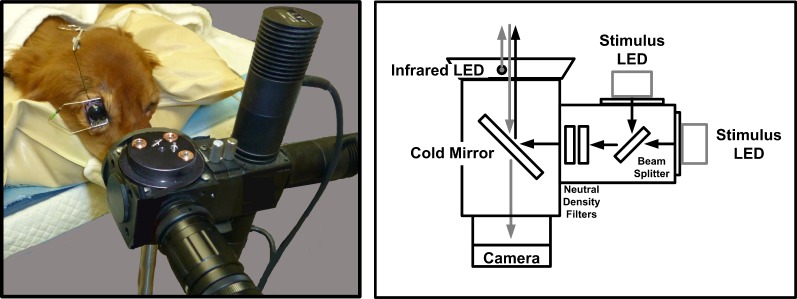
All recordings were done during the light phase of the daily cycle between 2 and 9 hours after the onset of the light phase. Dogs were kept in dim white light (0.9 lux) for at least 1 hour, including preparation time, and in complete darkness for 10 minutes before recording. For all PLR recording sessions, the dog was positioned in sternal recumbency, with the head placed on a deflatable cushion. The cornea of the right eye was anesthetized (0.5% proparacaine hydrochloride; Alcon Laboratories, Fort Worth, TX) and a lid speculum was inserted to ensure that the nictitating membrane and eyelids did not interfere with light exposure or visualization of the pupil. A small stay suture of 5-0 silk was placed in the bulbar conjunctiva on the central axis approximately 5 mm superior to the limbus to facilitate globe manipulation to maintain centration of the pupil on the optical axis of the recording apparatus. Suture ends were left long to be threaded between the outer surface of the eyelid and the lid speculum and clamped with a hemostat, which was draped over the top of the dog's head and taped to the deflatable cushion so as to maintain a constant pupil position throughout recording (Fig. 1). The eye was regularly lubricated with saline eye wash solution throughout the procedure.
Figure 1.
The camera and stimulus delivery portion of the PLR recording apparatus (left) and its schematic diagram (right). See Supplementary Material for full parts list. Photograph was taken with normal room light after the procedure.
Recordings were performed with a custom apparatus (Fig. 1) capable of timed delivery of a visible light stimulus from a high-power light-emitting diode (LED) (MCWHL2/white, M625L2/red, M470L2/blue; Thorlabs, Inc., Newton, NJ) and concurrent recording of pupil images at 30 frames per second using an infrared-sensitive camera (PC164CEX-2; Supercircuits Inc., Austin, TX) and continuous infrared illumination (880 nm LED). Stimulus delivery and image capture were synchronized via a data acquisition module and image acquisition card and controlled by custom software written in LabVIEW (National Instruments Corp., Austin, TX). The system design is based on that of a custom pupillometer intended for human application.25 The camera and LED stimulus delivery portion of the recording apparatus were mounted on a tripod and positioned 10 cm from the cornea (Fig. 1) with the pupil centered in the image frame. A piece of black fabric was draped over the dog's head and the camera throughout the procedure to ensure complete darkness. The direct PLR of the right eye was evaluated using a standardized protocol of 100-ms flashes of broad-spectrum white light presented in Newtonian view (4 cm stimulus diameter) at each of 10 intensities between 8 and 15 log photons/cm2/s (0.1 to 1400 lux). After dark-adaptation and positioning of the eye, the recording protocol required approximately 1 hour. If eye movements occurred during recording such that the entire pupil was no longer within the field of the camera, the position of the eye was adjusted as necessary between light stimuli using the conjunctival stay suture.
To investigate the response of intrinsically photosensitive retinal ganglion cells (ipRGCs), the duration and color of the stimuli were altered for a second set of flashes.3,26 PLRs were recorded in response to 1-second stimuli of blue light (470-nm peak; 14.5-nm half-width) and of red light (627-nm peak; 8.5-nm half-width). Both colored stimuli were tested at two intensities, 13.0 and 14.5 log photons/cm2/s (blue) and 13.5 and 15.0 log photons/cm2/s (red). The intensity of each red stimulus was increased by a half log unit over the intensity of the corresponding blue stimulus to correct for the reduced spectral sensitivity to red light. The blue stimuli should elicit PLRs mediated by ipRGCs (melanopsin; peak sensitivity approximately 480 nm) as well as rods and short-wavelength cones. The red light stimulus is outside the spectral sensitivity range of melanopsin and would be expected to stimulate primarily long wavelength-sensitive cones. Dogs have cones with peak sensitivities of approximately 429 and 555 nm. The spectral sensitivities of both types of cones are fairly broad and the long-wavelength cones are only approximately 0.7 log units less sensitive to stimuli at 625 nm than at 555 nm.17 Therefore, the 627-nm red light stimulus was more than adequate to stimulate these cones to initiate the PLR, particularly at the intensities used.
Irradiance (μW/cm2/nm) and illuminance (lux) measurements were performed using a National Institute of Standards and Technology–traceable calibrated spectroradiometer (ILT950; International Light Technologies, Peabody, MA) and irradiance values were subsequently converted to photon flux (photons/cm2/s) for the visible range. Pupil images were recorded for 5 seconds before each flash and for 15 to 85 seconds after the flash, depending on intensity of the light. Time between flashes ranged from 1 minute with the dimmest flash to 4 minutes with the brightest flash. The longer intervals between high-intensity stimuli were required to allow the pupil to fully return to baseline size before administering the next flash.
Baseline Pupil Measurements in Conscious Dogs
Four conscious, unrestrained dogs were dim-light and dark adapted as described above. Baseline pupil images were then captured using the infrared camera. Holding the camera by hand, a short series of pupil images could be captured without restraining the dogs.
Image Analysis
Pupil images were analyzed using batch processing in Photoshop (CS6 extended; Adobe Systems, Inc.; San Jose, CA). An “action” was created to calculate pupil area with a series of user-recorded steps (see Supplementary Material for detailed description). First, the pupil was selected by defining a pixel “color range” (Fig. 2A). The selection was then modified by the smooth command to eliminate extraneous pixels and then expanded and contracted by equal amounts to eliminate the hole in the selection caused by the reflection of light from the infrared LED off the surface of the cornea (Fig. 2B). A list of image frame number and corresponding pupil area was exported to a spreadsheet and used to calculate desired parameters (Fig. 3). Area measurements were converted from pixels to mm2 based on the known size of the lid speculum present in each pupil image (Fig. 2).
Figure 2.
Photoshop analysis showing selection of the pupil (red hash marks) based on pixel color (A). After expanding and contracting the selection by equal amounts, the hole in the selection caused by the infrared reflection is removed, leaving an accurate outline of the pupil (B). Red coloring was added to aid in visualizing the selection; hash marks are normally white.
Figure 3.
Pupillogram showing the characteristic change in pupil size after a 100-ms stimulus. PLR parameters of interest can be calculated as indicated.
PLR Parameters
For the studies described here, baseline pupil area was the average pupil area, in a dark-adapted dog, over a 1-second period before the light stimulus (Fig. 3). Baseline pupil diameter was calculated from area measurements assuming a round pupil. PLR constriction amplitude was defined as the difference between baseline pupil area and minimum pupil area attained following the light stimulus. These values were then converted to a percentage of baseline pupil area. PLR threshold was defined as the minimum stimulus intensity necessary to elicit a 5% pupil constriction. Latency was defined as the time between stimulus onset and the beginning of pupil constriction. Average constriction velocity was calculated as the constriction amplitude divided by the constriction time, where constriction time is calculated between the beginning of pupil constriction and the minimum pupil size.
Redilation of the pupil is biphasic with the initial redilation occurring more rapidly than the secondary redilation. Average redilation velocity was calculated for the initial redilation period and is defined as half the constriction amplitude divided by the time required for the pupil to redilate from its minimum size to half the baseline pupil size. Average sustained pupil area was calculated for the period between 10 and 40 seconds after light offset. The postillumination pupil response (PIPR)27,28 was then defined as the difference between baseline pupil area and sustained pupil area. These values were subsequently converted to percentage of the baseline pupil size.
ERG Evaluation
To assess retinal function, a standard bilateral ERG evaluation was performed in all dogs within 1 week following each PLR recording session. Before the recording session, both pupils were dilated with topical administration of 1% tropicamide. ERGs were elicited and recorded using a portable unit (HMsERG model 2000; RetVet Corp., Columbia, MO) as previously described.24 Each ERG session consisted of scotopic and photopic ERGs in accordance with procedures recommended by the European College of Veterinary Ophthalmology.21 Throughout a 20-minute period of dark adaptation, ERGs were recorded in response to 0.01 cd·s/m2 flashes (10.2 log photons/cm2/s) with 4 minutes of dark adaptation between recordings. Thereafter, scotopic responses to 3 cd·s/m2 and 10 cd·s/m2 flashes (12.65 and 13.20 log photons/cm2/s) were recorded.
In additional ERG recording sessions performed under dexmedetomidine restraint (35 μg/kg IM), a scotopic intensity series was performed in five dogs to determine the ERG scotopic threshold following 1 hour of dim-light adaptation and 10 minutes of complete dark adaptation, the same dark-adaptation procedure used in PLR recordings. The intensity series ranged from 0.03 mcd·s/m2 to 30 cd·s/m2 (7.65 to 13.65 log photons/cm2/s). Each recording session required approximately 1 hour. All ERG waveforms were evaluated for amplitude of the a- and b-waves. Threshold for the ERG recordings was defined as the minimum light intensity necessary to elicit a 10-μV b-wave amplitude. Threshold values for the ERG and PLR were compared using a two-tailed, heteroscedastic Student's t-test.
Results
Effects of Chemical Restraint Methods on the PLR
With injectable chemical restraint alone (methods CR1–CR4), large spontaneous fluctuations in pupil size occurred independent of light stimulation (Fig. 4). The frequency domain power spectra of the spontaneous pupillary fluctuations under conditions CR2, CR3, and CR4 were evaluated using fast Fourier transform (Matlab v.7.12.0; MathWorks, Inc., Natick, MA). Oscillations in baseline pupil size were composed primarily of components of less than 0.8 Hz. The most prominent of these components for all three restraint methods had a peak at 0.15 Hz, but no single frequency predominated in any of the spectra. No significant differences were found between the three restraint methods with respect to total power of pupillary fluctuations or in the frequency spectra of these flutuations.29,30 Pupillary fluctuations were further quantified by determining the mean pupil size during 20 seconds of baseline recording, calculating the deviation of each data point from this mean value, and computing the average deviation. There were no significant differences in this measure of baseline pupil size fluctuation among restraint methods CR2, CR3, and CR4, but these fluctuations were dramatically reduced with restraint method CR5 (Table 1). Range was calculated as the difference between the maximum and minimum pupil size during the 20-second baseline recording (Table 1). There were no significant differences in the range of amplitudes in baseline pupil sizes among restraint methods CR2, CR3, and CR4, but the magnitude of this range in baseline pupil size was greatly reduced with method CR5 (Table 1).
Figure 4.
Baseline pupil size with different chemical restraint protocols; dexmedetomidine and ketamine (method CR3) (A) and dexmedetomidine and butorphanol (method CR4) (B). PLR traces from a dog under high-dose dexmedetomidine restraint (method CR2) (C) and a dog under combined injectable and inhalant restraint (method CR5) (D). Pupil responses are shown as percent of baseline pupil area. No stimulus was used in traces (A) and (B). A 100-ms flash stimulus of 11 log photons/cm2/s was given 5 seconds into (C) and (D) recordings (dashed line), resulting in a large constriction. The substantial spontaneous fluctuation in pupil size observed under injectable chemical restraint (A–C) was completely eliminated with the use of inhalant restraint (D).
Table 1. .
Baseline Pupil Fluctuation as a Percent of Average Pupil Size for the Chemical Restraint Protocols Tested
|
Protocol |
No. of Trials |
Age, mo |
% Pupil Fluctuation, Average ± SD |
Range,* %, Average ± SD |
| CR2:dexmedetomidine, high dose | 5 | 6.8 ± 1.4 | 14.4 ± 1.8 | 49.0 ± 9.8 |
| CR3: dexmedetomidine/ketamine | 4 | 9.2 ± 0.5 | 10.6 ± 4.2 | 46.1 ± 0.2 |
| CR4:dexmedetomidine/butorphanol | 2 | 8.0 ± 1.5 | 13.6 ± 3.7 | 47.3 ± 1.5 |
| CR5: dexmedetomidine/isoflurane | 7 | 10 ± 0.1 | 0.8 ± 0.5 | 2.7 ± 1.3 |
Range is the difference between the maximum and minimum pupil size over a 20-second baseline recording. Low-dose dexmedetomidine (method CR1) did not allow for reliable recordings, making it impossible to quantify these measures.
Ventral rotation of the eye was also common for all chemical restraint protocols. This made visualization of the entire pupil for more than a few minutes difficult or impossible. In all cases, eyelid interference with imaging was a problem and necessitated the use of an eyelid speculum. However, low-dose dexmedetomidine (method CR1) did not provide adequate restraint to prevent the dog from resisting speculum placement. In addition, under this light chemical restraint, the eye was stationary for only very short periods, making it difficult to capture even a 10-second series of images.
With methods CR2 to CR5, it was possible to place an eyelid speculum to prevent eyelid interference with pupil visualization and to place a stay suture to prevent eye rotation. With methods CR2, CR3, and CR4, large spontaneous fluctuations in pupil size were observed that prevented reliable determinations of the PLR (Figs. 4A–C). With method CR5, on the other hand, very consistent baseline pupil sizes were obtained with minimal noise (Figs. 4, 5) and allowed for the use of an eyelid speculum and conjunctival stay suture for stable positioning of the eye without signs of stress in the animal.
Figure 5.
Baseline pupil behavior with different chemical restraint protocols. The dashed trace shows the fluctuating pupil size from a dog under dexmedetomidine restraint (CR2). The solid traces are from the same dog 3, 7, and 10 minutes after isoflurane administration was initiated and show the gradual progression to a stable baseline pupil.
The differences between injectable chemical restraint alone (methods CR1–CR4) and the addition of inhalant isoflurane (method CR5) were further illustrated by a trial in which pupil size was monitored after injectable chemical restraint alone and then at timed intervals after initiating isoflurane administration. The pupil was small and fluctuating in size with injectable restraint alone. Within 10 minutes of administering isoflurane, the pupil almost doubled in size and ceased fluctuating (Fig. 5).
PLR Recordings
Average baseline pupil diameter in conscious, unrestrained dogs was 10.06 ± 0.54 mm (mean ± SEM). Baseline pupil diameter under chemical restraint with method CR5 was 8.04 ± 0.16 mm, which was a significant reduction from that of awake dogs (P < 0.001). With a steady baseline pupil size under combined injectable and inhalant chemical restraint, it was possible to detect and reliably measure a PLR as small as 5% constriction. All PLRs recorded with method CR5 were characterized by four main phases, including the steady-state baseline pupil prior to the flash stimulus, the rapid pupil constriction in response to the stimulus, the initial redilation phase, and the slower secondary recovery to baseline pupil size (Fig. 6). As stimulus intensity was increased, the amplitude of pupil constriction also increased (Fig. 6, Table 2). Latency decreased with each increase of stimulus intensity. Average constriction velocity and initial redilation velocity increased with stimulus intensity between 8 and 11 log photons/cm2/s, but then declined slightly with each subsequent increase in intensity (Table 3). The kinetics of these responses are similar to those of PLRs recorded in people using a similar system.31 The PIPR was detectable with a stimulus of 11 log photons/cm2/s and increased with each subsequent increase in stimulus intensity (Table 3).
Figure 6.
PLR traces illustrating the range of pupil constriction observed in dogs under chemical restraint with method CR5 with 100-ms white light stimuli of increasing intensity (from top to bottom: 8.0, 8.5, 9.0, 13.0, and 15.0 log photons/cm2/s). Pupil responses are shown as percent of baseline pupil area. The stimulus onset occurred 5 seconds into each recording.
Table 2. .
PLR Constriction Amplitudes as a Function of Age and Stimulus Intensity
|
Stimulus Intensity, Log Photons/cm2/s |
Age, mo |
||||
| 4 | 6 | 8 | 10 | 12 | |
|
Constriction Amplitude, % | |||||
| 8 | 1.05 ± 0.61 | 1.24 ± 0.88 | 1.81 ± 1.21 | 1.36 ± 0.69 | 1.86 ± 1.5 |
| 8.5 | 11.94 ± 4.68 | 8.46 ± 2.95 | 5.92 ± 4.76 | 13.46 ± 5.21 | 7.6 ± 2.73 |
| 8.75 | 18.77 ± 6.33 | 17.26 ± 5.32 | 8.48 ± 6.1 | 24.7 ± 7.65 | 15.46 ± 4.3 |
| 9 | 28.67 ± 5.28 | 29.09 ± 5.19 | 22.3 ± 7.5 | 31.58 ± 6.37 | 26.64 ± 6.37 |
| 10 | 40.68 ± 3.05 | 42.2 ± 4.97 | 38.81 ± 6.35 | 43.06 ± 3.73 | 39.91 ± 3.23 |
| 11 | 50.51 ± 2.52 | 50.47 ± 3.76 | 49.69 ± 4.78 | 51.15 ± 2.88 | 49.52 ± 2.26 |
| 12 | 57.3 ± 2.56 | 55.73 ± 3.47 | 57.28 ± 4.3 | 57.85 ± 2.35 | 55.81 ± 1.81 |
| 13 | 58.81 ± 3.34 | 58.49 ± 3.18 | 63.01 ± 4.55 | 62.39 ± 3.47 | 59.87 ± 2.12 |
| 14 | 64.41 ± 5.23 | 61.71 ± 3.6 | 61.83 ± 5.13 | 63.28 ± 2.71 | 63.2 ± 3.32 |
| 15 | 65.55 ± 3.96 | 68.26 ± 3.78 | 68.67 ± 4.47 | 68.82 ± 3.4 | 63.9 ± 3.3 |
Data shown as percent of baseline pupil area (average ± SEM).
Table 3. .
PLR Parameters as a Function of Stimulus Intensity in Dogs Under CR5
|
Stimulus Intensity, Log Photons/cm2/s |
PLR Parameter, Average ± SEM |
|||
|
Latency, ms |
Constriction Speed, mm/s |
Redilation Speed, mm/s |
PIPR, % |
|
| 8 | 380.56 ± 26.66 | 0.5 ± 0.08 | 0.16 ± 0.02 | — |
| 8.5 | 328.47 ± 11.79 | 1.06 ± 0.14 | 0.38 ± 0.04 | — |
| 8.75 | 283.33 ± 8.95 | 1.47 ± 0.16 | 0.49 ± 0.05 | — |
| 9 | 265.28 ± 6.96 | 1.93 ± 0.15 | 0.66 ± 0.06 | — |
| 10 | 228.45 ± 3.74 | 2.54 ± 0.08 | 0.91 ± 0.06 | 4.72 ± 1.57 |
| 11 | 209.43 ± 2.98 | 2.82 ± 0.06 | 1.06 ± 0.06 | 7.29 ± 0.79 |
| 12 | 198.48 ± 3.86 | 2.69 ± 0.07 | 0.93 ± 0.06 | 17.38 ± 2.12 |
| 13 | 191.4 ± 3.78 | 2.53 ± 0.1 | 0.85 ± 0.06 | 26.77 ± 2.99 |
| 14 | 188.69 ± 5.08 | 2.54 ± 0.11 | 0.8 ± 0.07 | 30.71 ± 3.48 |
| 15 | 183.33 ± 3.16 | 2.28 ± 0.09 | 0.59 ± 0.05 | 44.4 ± 3.77 |
Values from all ages were averaged.
To assess repeatability of the PLR constriction amplitudes among the five recording sessions, Pearson product-moment correlation coefficients (r) were calculated for each dog. The average correlation coefficient for all dogs was 0.95 ± 0.02 (average ± SD) (P < 0.001), indicating a high rate of repeatability between recording sessions.
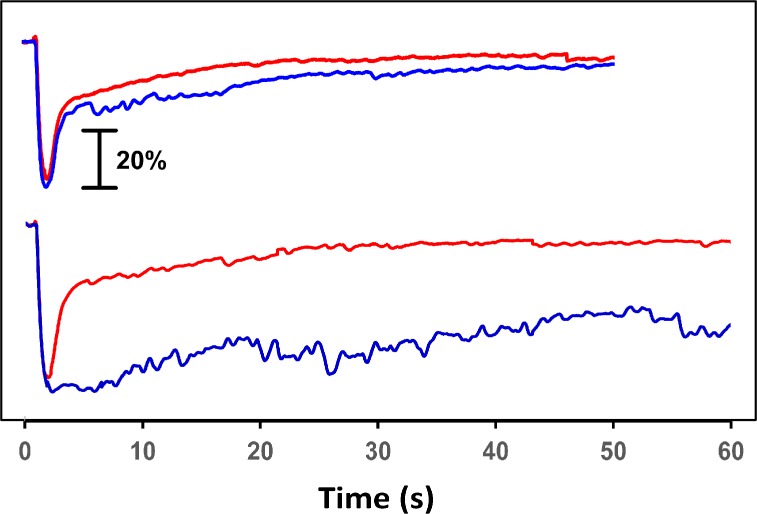
Red and blue stimuli resulted in robust PLRs with both intensities tested (Fig. 7). With stimuli of 13.0 and 13.5 log photons/cm2/s, the PLRs to blue and red light were very similar to one another for both the constriction and redilation phases. However, with stimuli of 14.5 and 15.0 log photons/cm2/s, blue and red light resulted in very different redilation phases. Whereas the pupil redilated quickly after offset of the red stimulus, pupil constriction persisted after offset of the blue stimulus (Fig. 7). This sustained constriction was present for at least 60 seconds after the stimulus.
Figure 7.
Blue and red traces show PLRs to 1-second stimuli of the corresponding color (top, 13.0 and 13.5 log photons/cm2/s; bottom, 14.5 and 15.0 log photons/cm2/s). The onset of each stimulus occurred 1 second into each recording. Recordings were performed in dogs using protocol CR5.
ERG and PLR Thresholds
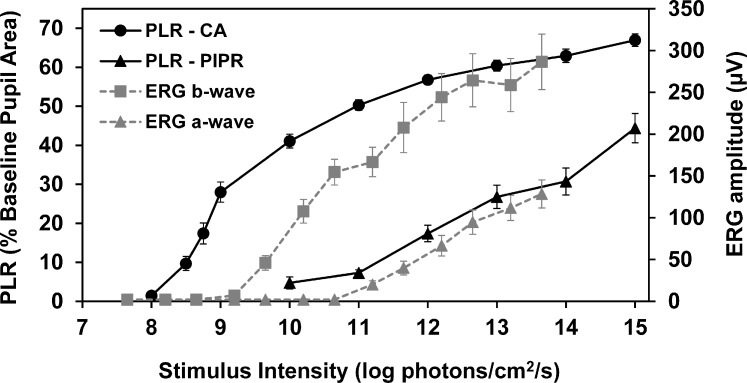
The ERG assessments demonstrated normal retinal function in all dogs throughout the 8-month period in which PLR recordings were obtained. The threshold stimulus intensity for the PLR was 8.59 ± 0.30 log photons/cm2/s (average ± SD), whereas the threshold was 9.56 ± 0.20 log photons/cm2/s for the ERG b-wave and 11.2 log photons/cm2/s for the ERG a-wave (Fig. 8 and Table 4). Thresholds for both the ERG and the PLR measures were defined as the minimum amplitude responses that were consistently larger than the amplitudes of baseline noise. For the PLR, threshold responses were constriction amplitudes of 5%. For the ERGs, threshold response amplitude was 10 μV. The stimulus intensity required to elicit a threshold ERG b-wave response was significantly higher by approximately 10 times compared with the stimulus required to produce a reliably detectable pupil constriction (P < 0.00005).
Figure 8.
Average PLR constriction amplitude (CA) and PIPR (both expressed as % baseline pupil area) (n = 7) and ERG a- and b-wave amplitudes (n = 5) in response to white light stimuli of increasing intensity. ERGs were recorded under dexmedetomidine restraint and PLRs were recorded using protocol CR5. Error bars represent SEM.
Table 4. .
ERG a- and b-Wave Amplitudes From the Scotopic Intensity Series
|
Stimulus Intensity |
ERG Amplitude, μV |
||
|
mcd·s/m2 |
Log Photons/cm2/s |
b-Wave |
a-Wave |
| 0.03 | 7.65 | ND | ND |
| 0.1 | 8.2 | ND | ND |
| 0.3 | 8.65 | ND | ND |
| 1 | 9.2 | 6.9 ± 3.4 | ND |
| 3 | 9.65 | 45.9 ± 8.8 | ND |
| 10 | 10.2 | 107.8 ± 14.3 | ND |
| 30 | 10.65 | 154.6 ± 15.5 | ND |
| 100 | 11.2 | 166.6 ± 17.6 | 20.1 ± 4.4 |
| 300 | 11.65 | 207.8 ± 30 | 39.8 ± 8.3 |
| 1000 | 12.2 | 244.1 ± 28.3 | 66.5 ± 12.4 |
| 3000 | 12.65 | 264.4 ± 31.8 | 94.8 ± 14.5 |
| 10000 | 13.2 | 258.7 ± 31.8 | 111.7 ± 15.1 |
| 30000 | 13.65 | 286.5 ± 33.1 | 128.5 ± 16.7 |
ND, not detectable.
Discussion
We have developed a recording system and protocol capable of reliably quantifying the PLR in the dog that can easily be adapted for use in other species. The system provides great flexibility with respect to stimulus color, duration, and range of intensity, allowing for thorough evaluation of many parameters known to affect the PLR. We have also demonstrated large spontaneous fluctuations in baseline pupil size when some forms of chemical restraint are used. Pupil size is determined by a balance of sympathetic and parasympathetic input to the iris. Disease states or trauma that affect this balance should be reflected in alterations in the PLR and/or in the oscillations in baseline pupil size. Therefore, the methods demonstrated in this study will be useful for characterizing the effects of disease and trauma on the neural pathways that regulate pupil size.
Using the appropriate stimulus conditions, we were also able to demonstrate ipRGC input to the canine PLR. Melanopsin, the ipRGC photopigment, has peak sensitivity at 480 nm, and these cells are slow to activate and have a high threshold.26 Below the ipRGC threshold, PLRs to blue and red light have been shown to be quite similar.28 However, when the ipRGCs are strongly activated, they cause a sustained pupil constriction persisting after light offset that is not seen with a red stimulus outside the melanopsin spectral sensitivity.26–28 We were able to replicate these findings (Fig. 7), suggesting that these characteristics of melanopsin input to the PLR are conserved in the dog.
Because of similarities in size and anatomy in canine and human eyes and the similarities between these species in neural pathways between the eye and brain, the dog is an excellent model for studying retinal-mediated responses to light stimuli and effects of neurological disease and trauma on pupil size regulation. Many diseases affecting pupil size regulation in people are recapitulated in dogs.32–34 This makes dogs very useful for evaluating potential therapeutic interventions to treat diseases that affect pupil size modulation. For most animal studies, assessment of visual system integrity is performed using ERG recordings. Obviously, the ERG assesses only the initial steps in the light-initiated neural processes that are mediated by the retina. This assessment tool provides no information in post–retinal processing of signals that originate in the retina. PLR measurements can provide such information. In addition, the sensitivity of the ERG performed with the recording electrode placed on the surface of the cornea is limited, due in large part to the distance between the recording electrode and the retina in which the electrical signals are generated.35,36 As a result, meaningful retinal function may still be present even when detectable ERG signals cannot be elicited.37 As our data illustrate, the threshold for the PLR (8.59 ± 0.30 log units) is much lower than that of the ERG (9.56 ± 0.20 log units). Consequently, quantitative PLR measurements may be able to detect residual retinal function and useful effects of therapeutic interventions that are too small to be detected with the ERG.37
Quantitative assessment of the PLR will enable sensitive detection of defects within the neural pathway that modulates pupil size. However, PLR recordings alone will not pinpoint the causes of any observed deficits. Accurate interpretation of the PLR data will require concurrent assessment of visual function using the ERG. Some disorders can affect the neural pathways involved in regulating pupil size without altering the image-forming and -processing functions of the retina and visual system.10–15 In these cases, when retinal function is normal as determined by ERG recordings, PLR abnormalities can be attributed to ipRCG dysfunction or neurological pathology outside of the retina. Alternatively, some diseases may affect the image-forming functions of the retina while the components of the PLR mediated by the ipRGCs are preserved. Combined ERG and evaluations of pupil size regulation can be used to identify and distinguish such disorders. With animal models it will be possible to associate alterations in the PLR and baseline pupil size oscillations with neuroanatomical localization of pathology.
It should be noted that the ERG and PLR were recorded under different forms of chemical restraint in this study. The conditions used for ERG recordings were those that have been well established and are currently in widespread use in veterinary clinical practice and research. It is unlikely that the current standards for chemical restraint used for ERG recordings will be modified. In addition, ERGs recorded in dogs under restraint with isoflurane are greatly reduced in amplitude compared with ERGs recorded in the same dogs restrained with dexmedetomidine.22 Based on these considerations, we attempted to obtain the PLR recordings using methods of chemical restraint similar to those used widely for ERG recordings. Unfortunately, as illustrated in our study, using the chemical restraint protocol employed for ERG recordings, we were unable to obtain a stable baseline pupil size. As a result, different protocols for chemical restraint will need to be used for ERG and PLR recordings in both research and clinical settings. Given this limitation, our conclusions about the differences in thresholds between the ERG and PLR are valid for the conditions under which these recordings can practically be made under standardized conditions.
The current study demonstrated a large difference among methods of chemical restraint with respect to baseline pupil size and spontaneous pupil fluctuation. Obtaining reliable PLR recordings using injectable chemical restraint alone was not possible due to spontaneous fluctuations in pupil size and movement of the eyes. For a person or animal in a relaxed state with a fixed visual focus, the pupil typically reaches a steady size determined by the balance of input from the sympathetic and parasympathetic systems.19 The observed pupil fluctuation therefore may indicate variable input from these two systems. In the normal dogs evaluated in this study, the frequencies and amplitudes of the fluctuations in baseline pupil size did not exhibit regular patterns. A study in cats found spontaneous pupil fluctuations that occurred only in lightly sedated or tranquilized animals and not in conscious or deeply anesthetized animals.38 Further evaluation found these fluctuations to be independent of sympathetic innervation but related to variations in parasympathetic outflow.38 These findings seem to be consistent with the fluctuations we observed; however, it would require further investigation to confirm the source of the fluctuations observed in the dogs. The balance between the sympathetic and parasympathetic inputs is likely to be altered in neurological diseases that affect the central pathways involved in modulating pupil size. Therefore, assessing these fluctuations in baseline pupil size under sedation may be a sensitive indicator of neurological disease.
In human PLR studies, isoflurane reduced the baseline pupil size and significantly depressed the constriction amplitude of the PLR.39 As the concentration of isoflurane was increased, pupil constriction was further suppressed with no detectable PLR at 1% end-tidal isoflurane.39 Although we did observe a reduction in baseline pupil size relative to unrestrained dogs, we regularly observed large PLR constriction amplitudes with the use of isoflurane. This difference from the human study could be due to differences in the duration of anesthesia, which has been shown to affect pupil size and responsiveness.40 Although our PLR measurements were completed within 1.5 hours of initiating isoflurane, the human study measured the PLRs 3 to 5 hours after initiating isoflurane. In addition, the PLRs observed in anesthetized dogs were very similar to those recorded in people who were not chemically restrained.31 Under the conditions of this investigation, chemical restraint using isoflurane did not appear to significantly alter the canine PLR.
For large animal recording, the system described here has several advantages over previously described methods for PLR assessment. The protocol we developed allows for stable positioning of the eye and full visualization of the pupil at all times. The average baseline pupil diameter achieved under anesthesia was similar to that reported in another dog study performed under medetomidine sedation (8.0 vs. 8.3 mm).41 However, the maximum pupil constriction achieved under medetomidine sedation was only 22% with a 0.2-second stimulus of 57 cd/m2 (equivalent to 285 cd·s/m2 or ∼14.6 log photons/cm2/s).41 In contrast, the protocol described here regularly achieved pupil constriction of greater than 60% using a comparable stimulus intensity. This constriction amplitude is similar to values achieved in awake human subjects using an analogous recording system and stimulus.25
The pupillography system used in these studies was constructed with readily available components and could be assembled by many research laboratories or could be commercialized for application in clinical veterinary ophthalmology and neurology. User control of stimulus characteristics and recording parameters allows great flexibility in probing various aspects of the visual system involved in the PLR and allows the system to be adapted for use in a variety of species. Varying the stimulus parameters also allows for the evaluation of relative contributions of the different retinal photopigments in initiating this reflex.27,28 Objective PLR analyses could also aid in localizing disease-related abnormalities in neurological and ophthalmic disorders.3
Supplementary Material
Acknowledgments
The authors thank Chathuri L. Daluwatte for technical assistance and Christine Sibigtroth and Melissa Carpentier for assistance with anesthesia.
Supported in part by Research to Prevent Blindness, Inc., the Batten Disease Support and Research Association, a University of Missouri Research Board grant, and Grant R01EY018815 from the National Institutes of Health.
Disclosure: R.E.H. Whiting, None; G. Yao, None; K. Narfström, None; J.W. Pearce, None; J.R. Coates, None; J.R. Dodam, None; L.J. Castaner, None; M.L. Katz, None
References
- 1. Wilhelm H. The pupil. Curr Opin Neurol. 2008; 21: 36–42 [DOI] [PubMed] [Google Scholar]
- 2. Scagliotti RH. Comparative neuro-ophthalmology. In: Gellat KN. ed Veterinary Ophthalmology. 3rd ed. Gainesville, FL: Lippincott Williams & Wilkins; 1999; 1307–1400 [Google Scholar]
- 3. Park JC, Moura AL, Raza AS, Rhee DW, Kardon RH, Hood DC. Toward a clinical protocol for assessing rod, cone, and melanopsin contributions to the human pupil response. Invest Ophthalmol Vis Sci. 2011; 52: 6624–6635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fotiou F, Fountoulakis KN, Goulas A, Alexopoulos L, Palikaras A. Automated standardized pupillometry with optical method for purposes of clinical practice and research. Clin Physiol. 2000; 20: 336–347 [DOI] [PubMed] [Google Scholar]
- 5. Yao G, Zhang K, Bellassai M, Chang B, Lei B. Ultraviolet light-induced and green light-induced transient pupillary light reflex in mice. Curr Eye Res. 2006; 31: 925–933 [DOI] [PubMed] [Google Scholar]
- 6. Thompson S, Whiting REH, Kardon RH, Stone EM, Narfstrom K. Effects of hereditary retinal degeneration due to a CEP290 mutation on the feline pupillary light reflex. Vet Ophthalmol. 2010; 13: 151–157 [DOI] [PubMed] [Google Scholar]
- 7. Lei B, Tullis GE, Kirk MD, Zhang K, Katz ML. Ocular phenotype in a mouse gene knockout model for infantile neuronal ceroid lipofuscinosis. J Neurosci Res. 2006; 1149: 1139–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thompson S, Stasheff SF, Hernandez J, et al. Different inner retinal pathways mediate rod-cone input in irradiance detection for the pupillary light reflex and regulation of behavioral state in mice. Invest Ophthalmol Vis Sci. 2011; 52: 618–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kankipati L, Girkin CA, Gamlin PD. The post-illumination pupil response is reduced in glaucoma patients. Invest Ophthalmol Vis Sci. 2011; 52: 2287–2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fotiou D, Stergiou V, Tsiptsios D, Lithari C, Nakou M, Karlovasitou A. Cholinergic deficiency in Alzheimer's and Parkinson's disease: evaluation with pupillometry. Int J Psychophysiol. 2009; 73: 143–149 [DOI] [PubMed] [Google Scholar]
- 11. Pittasch D, Behrens-Baumann W, Lobmann R, Lehnert H. Pupil signs of sympathetic autonomic neuropathy in patients with type 1 diabetes. Diabetes Care. 2002; 25: 1545–1550 [DOI] [PubMed] [Google Scholar]
- 12. Patwari PP, Stewart TM, Rand CM, et al. Pupillometry in congenital central hypoventilation syndrome (CCHS): quantitative evidence of autonomic nervous system dysregulation. Pediatr Res. 2012; 71: 280–285 [DOI] [PubMed] [Google Scholar]
- 13. Bremner FD, Smith SE. Pupil abnormalities in selected autonomic neuropathies. J Neuroophthalmol. 2006; 26: 209–219 [DOI] [PubMed] [Google Scholar]
- 14. Wilhelm BJ, Wilhelm H, Moro S, Barbur JL. Pupil response components: studies in patients with Parinaud's syndrome. Brain. 2002; 125: 2296–2307 [DOI] [PubMed] [Google Scholar]
- 15. De Seze J, Arndt C, Stojkovic T, et al. Pupillary disturbances in multiple sclerosis: correlation with MRI findings. J Neurol Sci. 2001; 188: 37–41 [DOI] [PubMed] [Google Scholar]
- 16. Bowmaker J, Dartnall H. Visual pigments of rods and cones in a human retina. J Physiol (Lond). 1980; 298: 501–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Neitz J, Geist T, Jacobs GH. Color vision in the dog. Vis Neurosci. 1989; 3: 119–125 [DOI] [PubMed] [Google Scholar]
- 18. Mowat FM, Petersen-Jones SM, Williamson H, et al. Topographical characterization of cone photoreceptors and the area centralis of the canine retina. Mol Vis. 2008; 14: 2518–2527 [PMC free article] [PubMed] [Google Scholar]
- 19. Lowenstein O, Lowenfeld IE. Mutual role of sympathetic and parasympathetic in shaping of the pupillary reflex to light. Arch Neurol and Psychiatry. 1950; 64: 341–377 [DOI] [PubMed] [Google Scholar]
- 20. Norman JC, Narfström K, Barrett PM. The effects of medetomidine hydrochloride on the electroretinogram of normal dogs. Vet Ophthalmol. 2008; 11: 299–305 [DOI] [PubMed] [Google Scholar]
- 21. Narfström K, Ekesten B, Rosolen SG, Spiess BM, Percicot CL, Ofri R. Guidelines for clinical electroretinography in the dog. Doc Ophthalmol. 2002; 105: 83–92 [DOI] [PubMed] [Google Scholar]
- 22. Lin S, Shiu W, Liu P, Cheng F, Lin Y, Wang W. The effects of different anesthetic agents on short electroretinography protocol in dogs. J Vet Med Sci. 2009; 71: 763–768 [DOI] [PubMed] [Google Scholar]
- 23. Katz ML, Narfström K, Johnson GS, O'Brien DP. Assessment of retinal function and characterization of lysosomal storage body accumulation in the retinas and brains of Tibetan terriers with ceroid-lipofuscinosis. Am J Vet Res. 2005; 66: 67–76 [DOI] [PubMed] [Google Scholar]
- 24. Katz M, Coates J, Cooper J, O'Brien DP, Jeong M, Narfstrom K. Retinal pathology in a canine model of late infantile neuronal ceroid lipofuscinosis. Invest Ophthalmol Vis Sci. 2008; 49: 2686–2695 [DOI] [PubMed] [Google Scholar]
- 25. Fan X, Miles JH, Takahashi N, Yao G. Sex-specific lateralization of contraction anisocoria in transient pupillary light reflex. Invest Ophthalmol Vis Sci. 2009; 50: 1137–1144 [DOI] [PubMed] [Google Scholar]
- 26. Gamlin PDR, Mcdougal DH, Pokorny J, Smith VC, Yau K, Dacey DM. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res. 2007; 47: 946–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kankipati L, Girkin C, Gamlin P. Post-illumination pupil response in subjects without ocular disease. Invest Ophthalmol Vis Sci. 2010; 51: 2764–2769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Markwell E, Feigl B, Zele A. Intrinsically photosensitive melanopsin retinal ganglion cell contributions to the pupillary light reflex and circadian rhythm. Clin Exp Optometry. 2010; 93: 137–149 [DOI] [PubMed] [Google Scholar]
- 29. Lüdtke H, Wilhelm B, Adler M, Schaeffel F, Wilhelm H. Mathematical procedures in data recording and processing of pupillary fatigue waves. Vision Res. 1998; 38: 2889–2896 [DOI] [PubMed] [Google Scholar]
- 30. Phillips M a, Szabadi E, Bradshaw CM Comparison of the effects of clonidine and yohimbine on spontaneous pupillary fluctuations in healthy human volunteers. Psychopharmacology. 2000; 150: 85–89 [DOI] [PubMed] [Google Scholar]
- 31. Fan X, Miles J, Takahashi N, Yao G. Abnormal transient pupillary light reflex in individuals with autism spectrum disorders. J Autism Dev Disord. 2009; 39: 1499–1508 [DOI] [PubMed] [Google Scholar]
- 32. Baehr W, Frederick JM. Naturally occurring animal models with outer retina phenotypes. Vision Res. 2009; 49: 2636–2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Casal M, Haskins M. Large animal models and gene therapy. Eur J Hum Genet. 2006; 14: 266–272 [DOI] [PubMed] [Google Scholar]
- 34. Sargan DR. IDID: inherited diseases in dogs: web-based information for canine inherited disease genetics. Mamm Genome. 2004; 15: 503–506 [DOI] [PubMed] [Google Scholar]
- 35. Bui B, Weisinger H, Sinclair A, Vingrys A. Comparison of guinea pig electroretinograms measured with bipolar corneal and unipolar intravitreal electrodes. Doc Ophthalmol. 1998; 95: 15–34 [DOI] [PubMed] [Google Scholar]
- 36. Derwent JJK, Padnick-Silver L, McRipley M, Giuliano E, Linsenmeier RA, Narfstrom K. The electroretinogram components in Abyssinian cats with hereditary retinal degeneration. Invest Ophthalmol Vis Sci. 2006; 47: 3673–3682 [DOI] [PubMed] [Google Scholar]
- 37. Melillo P, Pecchia L, Testa F, Rossi S, Bennett J, Simonelli F. Pupillometric analysis for assessment of gene therapy in Leber congenital amaurosis patients. Biomed Eng Online. 2012; 11: 40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Borgdorff P. Respiratory fluctuations in pupil size. Am J Physiol. 1975; 228: 1094–1102 [DOI] [PubMed] [Google Scholar]
- 39. Larson M, Sessler D, McGuire J, Hynson J. Isoflurane, but not mild hypothermia, depresses the human pupillary light reflex. Anesthesiology. 1991; 75: 62–67 [DOI] [PubMed] [Google Scholar]
- 40. Tayefeh F, Larson MD, Sessler DI, Eger EI, Bowland T. Time-dependent changes in heart rate and pupil size during desflurane or sevoflurane anesthesia. Anesth Analg. 1997; 85: 1362–1366 [DOI] [PubMed] [Google Scholar]
- 41. Grozdanic SD, Matic M, Sakaguchi DS, Kardon RH. Evaluation of retinal status using chromatic pupil light reflex activity in healthy and diseased canine eyes. Invest Ophthalmol Vis Sci. 2007; 48: 5178–5183 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.