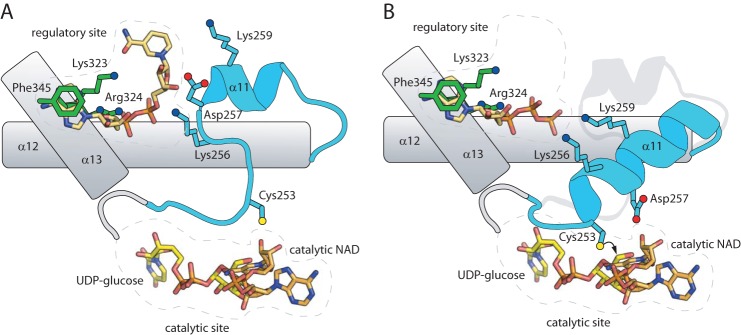
FIGURE 7.
Conceptual model for Ugd regulation. A, in the presence of high concentrations of UDPGA or NAD (shown), these molecules can bind in the regulatory site. Through interactions with residues such as Lys-256 and Asp-257, the mobile loop, including α11, is stabilized in a conformation where Cys-253 is misplaced from the catalytic site, resulting in a conformation that is not competent for turnover (this conformation is seen in UDPGA-bound UgdKp, PDB 3PJG (11)). B, if a nucleotide triphosphate is bound in this site (ATP depicted), then interactions mediated by the γ-phosphate with residues on α11 (most likely Lys-256 and Lys-259) stabilize this region as a long α-helix in an alternative conformation. This conformation is seen in NAD plus UDP-bound UgdKp (PDB 3PLR (11)), except that ATP was modeled in analogy to the second UDPGA in 3PJG. In this conformation, Cys-253 is correctly positioned for attacking the aldehyde moiety of UDPG, and catalysis can proceed. Note that this conformation is likely also stable in the absence of NTP binding, as catalysis is not dependent upon the presence of NTPs under low NAD concentrations.