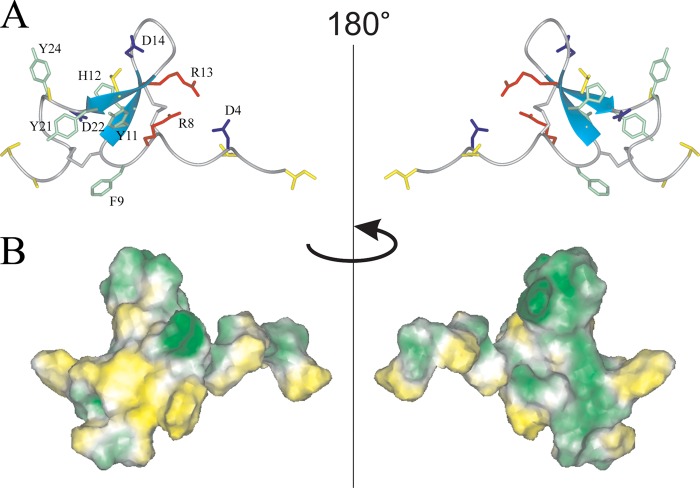
FIGURE 5.
Two-sided view of the determined spatial structure of Ugr 9-1. A, ribbon representation. Positively and negatively charged side chains are shown in red and blue, respectively. The aromatic and hydrophobic (Leu, Ile, Val, Thr, and Ala) side chains are shown in green and yellow, and cysteine side chains in gray. B, hydrophobicity of the peptide surface. The peptide surface is colored from yellow (hydrophobic) to green (hydrophilic), according to the molecular hydrophobic potential (49).